Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven
Get insights from 115 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
Correct option is (iii)
Nitrogen gas has a complete octet structure for both atoms and is unreactive due to the presence of a strong triple bond. On the other hand, phosphor has bonds with unstable angles strains compared to nitrogen, therefore it burns quickly, thus readily reacts. Nitrogen has a lower electron gain enthalpy than phosphorus.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i) and (ii)
(i) The modest dispersion force causes attraction in noble gases.
(ii) In nature, xenon fluorides are reactive.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (ii) and (iii)
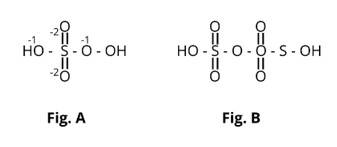
Here fig (b) contains one S-S bond.
The oxidising behaviour of H2SO4 is represented by (ii) and (iii) among the aforementioned four. It oxidises HI in reaction (ii) and then reduces to SO2.
The oxidation state of sulphur's core atom drops from +6 to +4. It oxidises copper in (iii) and is reduced to SO2.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i)
Oxidation state of S=+6
(iii) Iron oxide with K2O and Al2O3 is used to increase the rate of attainment of equilibrium in Haber's process.
(iv) Change in enthalpy is negative for the preparation of SO3 by catalytic oxidation of SO2.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i) and (iv)
(i) As2O3 < SiO2 < P2O3 < SO2 is the order of acid
(ii) AsH3 < PH3 < NH3 is the correct or enthalpy of vapourization
(iii) S < O < F < Cl is the correct order of more negative gain enthalpy
(iv) H2O > H2Se > H2Te is the order of thermal stability
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii) and (iv)
The P4 molecule in white phosphorus has an angular strain, white phosphorus is very reactive.
PCl5 is ionic in the solid state, with a tetrahedral cation and an octahedral anion.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i) and (iii)
SO2 is employed as an antichlor, disinfectant, and preservative in the bleaching of wool and silk.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i)
Due to its high electronegativity and tiny size, F is the only interhalogen with weaker X-X' bonds and no X-X bonds.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (ii) and (iv)
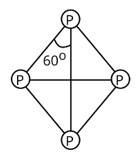
Tetrahedral geometry characterises the structure of a white phosphorus molecule.
Phosphorus has a valency of 5, and there are four phosphorus atoms in total. As a result, there will be a total of 4*5=20 valence electrons.
As a result, each P atom will have one lone pair of electrons, and each covalent bond (two electrons) will be established.
In a molecule of white phosphorus, there will be four lone pairs of electrons and six P-P single bonds in total.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (ii), (iii)
MI > MBr > MCl > MF is an iconic character of metal halide
F2 > Cl2 > Br2 > I2 is a bond dissociation enthalpy
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
