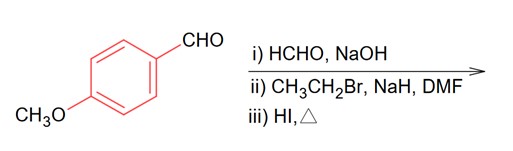Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven The p-Block Elements provides well-structured answers covering all the key terminologies of the chapter. The chapter focuses on The p-block Elements. It emphasizes the chemistry of group 15 (nitrogen family), group 16 (oxygen family), group 17 (halogens), and group 18 (noble gases). It includes Multiple-Choice Questions, Short Answer Type Questions, Very Short Answer Type Questions, and Long Answer Type Questions. By practicing these questions, the students will deepen their understanding of concepts such as the anomalous behavior of the first element of each group, oxidation states, the structure of important compounds, and the basic nature of oxides.
Students should download the P Block NCERT PDF from this page and read it as per their schedule. It will help them understand and memorize all the concepts and boost their confidence for appearing in the exam. The PDF is prepared by the subject matter expert. It is a highly reliable and accurate practice material. These questions help students to prepare for the CBSE Board exams and entrance exams like NEET and JEE.
- NCERT Exemplar Class 12 Chemistry The p-Block Elements Short Answer Type Questions
- NCERT Exemplar Class 12 Chemistry Chapter 7 Long Answer Type Questions
- NCERT Exemplar Class 12 Chemistry The p-Block Elements Matching Type Questions
- NCERT Exemplar Class 12 Chemistry Chapter 7 Assertion and Reason Type Questions
- NCERT Exemplar Class 12 Chemistry The p-Block Elements Objective Type Questions
- 26th February 2021 (Second Shift)
NCERT Exemplar Class 12 Chemistry The p-Block Elements Short Answer Type Questions
See Below SA Type Solutions
Q: In the preparation of H2SO4 by Contact Process, why is SO3 not absorbed directly in water to form H2SO4?
A: This is a short answer type question as classified in NCERT Exemplar
SO3 generates a dense fog of sulphuric acid that does not condense quickly, it is not absorbed directly in water to form H2SO4.
PH3 is insoluble in water and cannot create hydrogen bonds with it, it forms bubbles, whereas NH3 dissolves because it is soluble in water and can form hydrogen bonds with it.
Read more:
Commonly asked questions
Explain why the stability of oxoacids of chlorine increases in the order given below:
HClO < HClO2 < HClO3 < HClO4
This is a short answer type question as classified in NCERT Exemplar
Oxygen is more electronegative than chlorine, the negative charge on chlorine disperses from ClO− to ClO4− ion as the number of oxygen atoms linked to chlorine grows. As a result, ion stability will improve in the following order:
As the stability of the conjugate base rises, the acidic strength of the corresponding acid increases in the following order:
HClO < HClO2 < HClO3 < HClO4
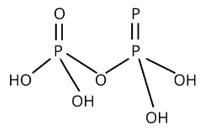
Write the structure of pyrophosphoric acid.
This is a short answer type question as classified in NCERT Exemplar
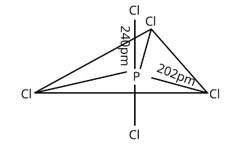
In PCl5 , phosphorus is in sp3d hybridised state but all its five bonds are not equivalent. Justify your answer with reason.
This is a short answer type question as classified in NCERT Exemplar
Three P—Cl bonds are arranged in one plane at a 1200 angle. These bonds are known as equatorial bonds because they are formed between two points. The remaining two P—Cl bonds, one above the equatorial plane and the other below it, form 90° angle with the plane. Axial bonds are the name for these types of bonds. Because axial bond pairs are subjected to greater repulsive interaction than equatorial bond pairs, axial bonds are slightly longer and hence slightly weaker than equatorial bonds, making the PCl5 molecule more reactive.
Why is nitric oxide paramagnetic in gaseous state but the solid obtained on cooling it is diamagnetic?
This is a short answer type question as classified in NCERT Exemplar
NO2 exists as a monomer with one unpaired electron in the gaseous state, but it dimerises to N2O4 in the solid state, leaving no unpaired electron, making the solid form diamagnetic.
Give reason to explain why ClF3 exists but FCl3 does not exist.
This is a short answer type question as classified in NCERT Exemplar
Chlorine has vacant d-orbitals, which become excited upon bonding when electrons from the 3p-orbital are promoted to the 3d-orbital, giving it a covalency of three.
Due to the lack of unoccupied d-orbitals in the second energy shell, fluorine cannot expand its octet. As a result, it can only have one covalency.
Out of H2O and H2S, which one has higher bond angle and why?
This is a short answer type question as classified in NCERT Exemplar
Oxygen is more electronegative than sulphur, the bond angle of H2O is greater, and the bod pair electron of an OH bond will be closer to oxygen, causing bond-pair repulsion between bond pairs of two OH bonds
SF6 is known but SCl6 is not. Why?
This is a short answer type question as classified in NCERT Exemplar
Six F atoms can be accommodated around sulphur due to fluorine's tiny size, however the chloride ion is comparably bigger, resulting in interatomic repulsion.
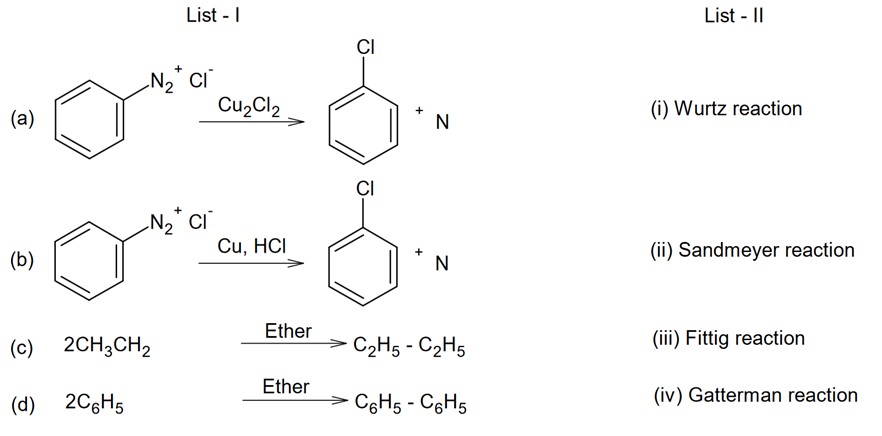
In reaction with Cl2 , phosphorus forms two types of halides ‘A’ and ‘B’. Halide A is yellowish-white powder but halide ‘B’ is colourless oily liquid. Identify A and B and write the formulas of their hydrolysis products.
This is a short answer type question as classified in NCERT Exemplar
A is PCl5 (yellowish white powder)
P4 + 10Cl2→4PCl5
B is PCl3 (colourless oily liquid)
Hydrolysis of the following product
PCl3+3H2O→H3PO3+3HCl
PCl5+4H2O→H3PO4+5HCl
In the ring test of NO3- ion, Fe2+ ion reduces nitrate ion to nitric oxide, which combines with Fe2 + (aq) ion to form brown complex. Write the reactions involved in the formation of brown rings.
This is a short answer type question as classified in NCERT Exemplar
NO3− + 3Fe2+ + 4H+→ NO+ + 3Fe3+ + 2H2O
[Fe (H2O)6]2+ + NO → [Fe (H2O)5 (NO)]2+ + H2O ( brown ring )
Explain why ozone is thermodynamically less stable than oxygen?
This is a short answer type question as classified in NCERT Exemplar
Due its disintegration into oxygen results in the release of heat ΔH is negative) and an increase in entropy (ΔS is positive), ozone is thermodynamically unstable in comparison to oxygen. These two reactions combine to produce a substantial negative Gibbs energy change (ΔG) for its conversion to oxygen.
P4O6 reacts with water according to equation P4O6 + 6H2O→ 4H3PO3. Calculate the volume of 0.1 M NaOH solution required to neutralise the acid formed by dissolving 1.1 g of P4O6 in H2O.
This is a short answer type question as classified in NCERT Exemplar
P4O6 + 6H2O→4H3PO3 (i)
Next is neutralization
4 times H3PO3 + 2NaOH→Na2HPO3 + 2H2O (ii)
On addition of 2 equations
P4O6+8NaOH→4Na2HPO3+2H2O
P4O6 ( mol.mass )= (4×31+16×6)=220
Number of moles of P4O6 = =
The product formed is neutralized 8 moles of NaOH
P4O6 = 8× = molNaOH
Molarity of NaOH in 1 litre = 0.1M
Molarity =
Volume =
= × = 0.4 L
White phosphorus reacts with chlorine and the product hydrolyses in the presence of water. Calculate the mass of HCl obtained by the hydrolysis of the product formed by the reaction of 62 g of white phosphorus with chlorine in the presence of water
This is a short answer type question as classified in NCERT Exemplar
P4+6Cl2→4PCl3… (i)
PCl3+3H2O→H3PO3+3HCl ×4
On adding eq. (i) and (ii)
P4+6Cl2+12H2O→4H3PO3+12HCl
1 mol of white phosphorus produces 12 mol of HCl
62 g of white phosphorus has been taken which is equivalent to = mol. Therefore 6 molHCl will be formed.
Mass of 6 molHCl=6×36.5=219.0g HCl
Name three oxoacids of nitrogen. Write the disproportionation reaction of that oxoacid of nitrogen in which nitrogen is in +3 oxidation state.
This is a short answer type question as classified in NCERT Exemplar
Three oxoacids are
(a) HNO2, Nitrous acid
(b) HNO3, Nitric acid
(c) Hyponitrous acid, H2N2O2
3HNO2![]() + H2O + 2NO
+ H2O + 2NO
Nitric acid forms an oxide of nitrogen on reaction with P4O10. Write the reaction involved. Also write the resonating structures of the oxide of nitrogen formed.
This is a short answer type question as classified in NCERT Exemplar
On interaction with P4O10 forms an oxide of nitrogen, N2O5, and metaphosphoric acid, 3, nitric acid is formed. HPO3
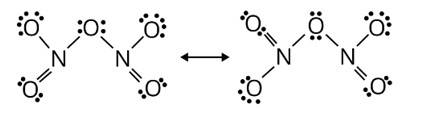
HNO3 + P4O10→4HPO3 + 2N2O5
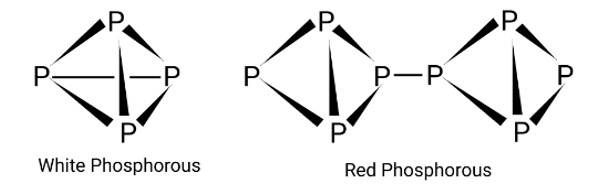
Phosphorus has three allotropic forms — (i) white phosphorus (ii) red phosphorus and (iii) black phosphorus. Write the difference between white and red phosphorus since their structure and reactivity.
This is a short answer type question as classified in NCERT Exemplar
The crystal structure of red phosphorus features a sophisticated network of bonding, whereas white phosphorus is made up of P4 molecules. To avoid natural combustion, white phosphorus must be stored in water, but red phosphorus is stable in air.
Give an example to show the effect of concentration of nitric acid on the formation of oxidation products.
This is a short answer type question as classified in NCERT Exemplar
When nitric acid is mixed with copper metal, it produces distinct oxidation products.
3Cu + 8HNO3 (dil.)→3Cu (NO3)2 + 2NO + 4H2O
Cu + 4HNO3 (Conc)→3Cu (NO3)2 + 2NO2 + 2H2O
PCl5 reacts with finely divided silver on heating and a white silver salt is obtained, which dissolves on adding excess aqueous NH3 solution. Write the reactions involved to explain what happens.
This is a short answer type question as classified in NCERT Exemplar
PCl5 + 2Ag→2AgCl + PCl3
AgCl + 2NH3 (aq)→ [Ag (NH3)2] + Cl-
Phosphorus forms several oxoacids. Out of these oxoacids phosphinic acid has strong reducing properties. Write its structure and write a reaction showing its reducing behaviour
This is a short answer type question as classified in NCERT Exemplar
The following reaction with silver nitrate demonstrates phosphinic acid reducing behaviour:
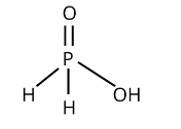
4AgNO3 + 2H2O + H3PO2→4Ag + 4HNO3 + H3PO4
Write a balanced chemical equation for the reaction showing catalytic oxidation of NH3 by atmospheric oxygen.
This is a short answer type question as classified in NCERT Exemplar
4NH3 + 5O2 ![]() 4NO + 6H2O
4NO + 6H2O
NCERT Exemplar Class 12 Chemistry Chapter 7 Long Answer Type Questions
Following Are LA Solutions
| Q: On heating compound (A) gives a gas (B) which is a constituent of air. This gas when treated with 3 mol of hydrogen (H2) in the presence of a catalyst gives another gas (C) which is basic in nature. Gas C on further oxidation in moist condition gives a compound (D) which is a part of acid rain. Identify compounds (A) to (D) also give necessary equations of all the steps involved. |
| Ans. A = NH4NO2 B = N2 C = NH3 D = HNO3 (i) NH4NO2→N2 + 2H2O (ii) N2 + 3H2→2NH3 (iii) 4NH3 + 5O2→4NO + 6H2O 4NO + O2→4NO2 |
Also read:
Commonly asked questions
An amorphous solid “A” burns in air to form a gas “B” which turns lime water milky. The gas is also produced as a by-product during roasting of sulphide ore. This gas decolourises acidified aqueous KMnO4 solution and reduces Fe3+ to Fe2+ . Identify the solid “A” and the gas “B” and write the reactions involved.
This is a long answer type question as classified in NCERT Exemplar
‘A’ is S8 ‘B’ is SO2 gas
S8 + 8O2 ![]() 8SO2
8SO2
2MnO4 – + 5SO2 + 2H2O → 5 SO42– + 4H+ + 2Mn2+
(violet) (colourless)
2Fe3+ + SO2 + 2H2O → 2Fe2+ + SO42– + 4H+
On heating lead (II) nitrate gives a brown gas “A”. The gas “A” on cooling changes to colourless solid “B”. Solid “B” on heating with NO changes to a blue solid ‘C’. Identify ‘A’, ‘B’ and ‘C’ and write reactions involved and draw the structures of ‘B’ and ‘C’.
This is a long answer type question as classified in NCERT Exemplar
Here, ‘A’ is NO2 (Nitrogen dioxide)
‘B’ is N2O4 (dinitrogen tetraoxide)
‘C’ is N2O3 (dinitrogen trioxide)
A brown gas is produced when lead nitrate (II) is heated
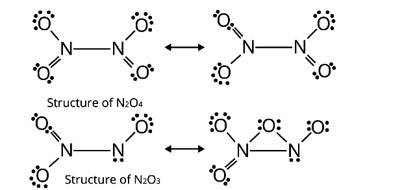
2Pb (NO3)2 ![]() 2PbO + 4NO2 + O2
2PbO + 4NO2 + O2
2NO2 ![]() N2O4
N2O4
2NO + N2O4  2N2O3
2N2O3
NCERT Exemplar Class 12 Chemistry The p-Block Elements Matching Type Questions
See Below Matching Type Questions
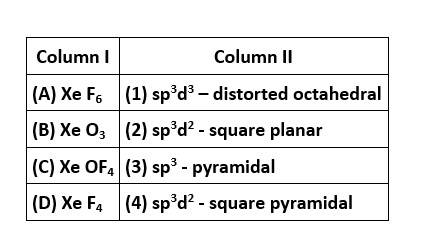
Correct option is (i)
In XeF6, the central Xe atom undergoes sp3d3 hybridisation and the molecular geometry is distorted octahedral with 1 lone pair and 6 bond pairs of electrons.
In XeO3, the central Xe atom undergoes sp3 hybridisation and the molecular geometry is pyramidal with 1 lone pair and 3 bonding domains of electrons.
In XeOF4, the central Xe atom undergoes sp3d2 hybridization and the molecular geometry is square pyramidal with 1 lone pair and 5 bond pairs of electrons.
In XeF4, the central Xe atom undergoes sp3d2 hybridization and the molecular geometry is square planar with 2 lone pairs and 4 bond pairs of electrons.
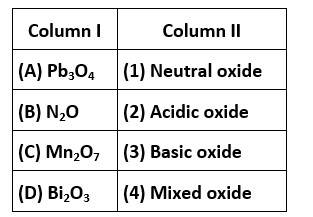
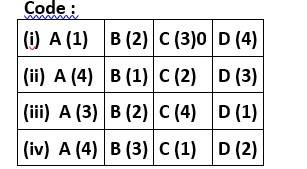
Correct option is (ii)
A. Pb3O4is a mixed ore
B. N2O is a neutral oxide
C. Mn2O7is acidic oxide
D. Bi2O3is basic oxide
To deepen their understanding of this chapter, students should also practice - NCERT Solutions for Class 12 Chemistry P Block Elements.
Commonly asked questions
Match the items of Columns I and II and mark the correct option.
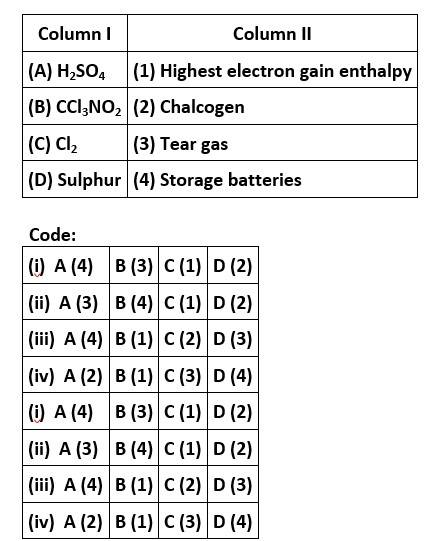
This is a matching answer type question as classified in NCERT Exemplar
Correct option is (i)
CCl3NO2 stands for chloropicrin, which is known as tear gas. Cl is the element of the highest electron gain enthalpy. Sulphur is the element belonging to 16th group, also known as chalcogens.
Match the species given in Column I with the shape given in Column II and mark the correct option.
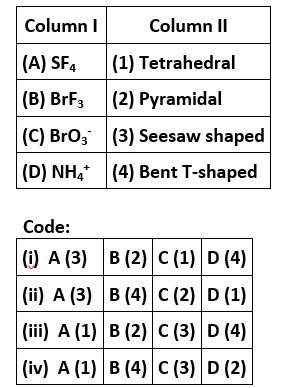
This is a matching answer type question as classified in NCERT Exemplar
Correct option is (ii)
Match the items of Columns I and II and mark the correct option.
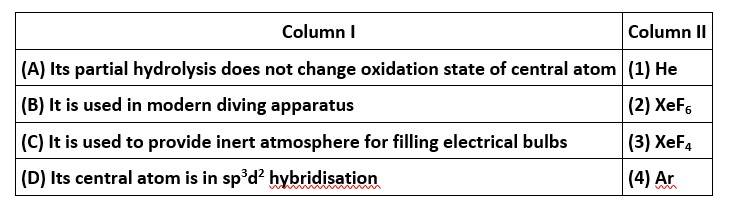
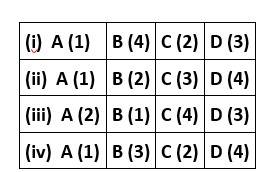
This is a matching answer type question as classified in NCERT Exemplar
Correct option is (iii)
NCERT Exemplar Class 12 Chemistry Chapter 7 Assertion and Reason Type Questions
See Below Assertion and Reason Type Questions
Assertion: N2 is less reactive than P4.
Reason: Nitrogen has more electron gain enthalpy than phosphorus.
(i) Both assertion and reason are correct statements, and reason is the correct explanation of the assertion.
(ii) Both assertion and reason are correct statements, but reason is not the correct explanation of the assertion.
(iii) Assertion is correct, but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Both assertion and reason are wrong statements.
Correct option is (iii) Nitrogen gas has a complete octet structure for both atoms and is unreactive due to the presence of a strong triple bond. On the other hand, phosphor has bonds with unstable angles strains compared to nitrogen, therefore it burns quickly, thus readily reacts. Nitrogen has a lower electron gain enthalpy than phosphorus.
Reason : HNO3 forms a protective layer of ferric nitrate on the surface of iron.
(i) Both assertion and reason are correct statements, and reason is the correct explanation of the assertion.
(ii) Both assertion and reason are correct statements, but reason is not the correct explanation of the assertion.
(iii) Assertion is correct, but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Both assertion and reason are wrong statements.
Correct option is (iii)
HNO3 forms an oxide layer on the surface of iron. This is also known as corrosion or rusting.
Commonly asked questions
Assertion : HI cannot be prepared by the reaction of KI with concentrated H2SO4
Reason : HI has lowest H–X bond strength among halogen acids
(i) Both assertion and reason are correct statements, and reason is the correct explanation of the assertion.
(ii) Both assertion and reason are correct statements, but reason is not the correct explanation of the assertion.
(iii) Assertion is correct, but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Both assertion and reason are wrong statements.
This is a assertion and reason answer type question as classified in NCERT Exemplar
Correct option is (ii)
Because H2SO4 is a stronger oxidising agent and HI is a stronger reducing agent, H2SO4 oxidizes and is reduced to SO2.
Assertion : Both rhombic and monoclinic sulphur exist as S8 but oxygen exists as O2 .
Reason : Oxygen forms pπ – pπ multiple bonds due to small size and small bond length Pπ- Pπ bonding is not possible in sulphur.
(i) Both assertion and reason are correct statements, and reason is the correct explanation of the assertion.
(ii) Both assertion and reason are correct statements, but reason is not the correct explanation of the assertion.
(iii) Assertion is correct, but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Both assertion and reason are wrong statements.
This is a assertion and reason answer type question as classified in NCERT Exemplar
Correct option is (i)
The rhombic and the monoclinic forms of sulphur exist as S8 . Oxygen forms a p\pi - p\pi multiple bond due to its small size and short bond length, but Pπ- Pπ bonding is not possible because of sulphur's larger atomic size.
Assertion : NaCl reacts with concentrated H2SO4 to give colourless fumes with pungent smell. But on adding MnO2 the fumes become greenish yellow.
Reason : MnO2 oxidises HCl to chlorine gas which is greenish yellow.
(i) Both assertion and reason are correct statements, and reason is the correct explanation of the assertion.
(ii) Both assertion and reason are correct statements, but reason is not the correct explanation of the assertion.
(iii) Assertion is correct, but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Both assertion and reason are wrong statements.
This is a assertion and reason answer type question as classified in NCERT Exemplar
Correct option is (i)
When NaCl reacts with concentrated H2SO4, it produces colourless fumes with a pungent smell, but when MnO2 is added, the fumes turn greenish yellow. MnO2 oxidizes HCl to chlorine gas, which then turns greenish yellow.
Assertion : SF6 cannot be hydrolysed but SF4 can be.
Reason : Six F atoms in SF6 prevent the attack of H2O on sulphur atom of SF6
(i) Both assertion and reason are correct statements, and reason is the correct explanation of the assertion.
(ii) Both assertion and reason are correct statements, but reason is not the correct explanation of the assertion.
(iii) Assertion is correct, but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Both assertion and reason are wrong statements.
This is a assertion and reason answer type question as classified in NCERT Exemplar
Correct option is (i)Here, the S atom is surrounded by 6F atoms, so it's complected for H2O to attack SF6, but in case of SF4, it's surrounded by 4F atoms, so it's to attack H2O.
NCERT Exemplar Class 12 Chemistry The p-Block Elements Objective Type Questions
Follow Are MCQs
On addition of conc. H2SO4 to a chloride salt, colourless fumes are evolved but in case of iodide salt, violet fumes come out. This is because
(i) H2SO4 reduces HI to I2
(ii) HI is of violet colour
(iii) HI gets oxidised to I2
(iv) HI changes to HI2O3
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
Hydrogen iodide is a more powerful reducing agent than sulphuric acid, it reduces the amount of iodine in the solution from H2SO4 to SO2 and HI to I2
When chloride salts are treated with sulfuric acid, HCl gas is formed, which produces a colourless gas.
NaCl + H2SO4→HCl + Na2SO4
Violet fumes are produced during the reaction due to the creation of iodine (I2) gas.
2NaI + H2SO4→Na2SO4 + 2HI2H2O + SO2 + I2
In qualitative analysis when H2S is passed through an aqueous solution of salt acidified with dil. HCl, a black precipitate is obtained. On boiling the precipitate with dil. HNO3, it forms a solution of blue colour. Addition of excess of aqueous solution of ammonia to this solution gives _________.
(i) Deep blue precipitate of Cu(OH)2
(ii) Deep blue solution of [Cu(NH3)4]2+
(iii) Deep blue solution of Cu(NO3)2
(iv) Deep blue solution of Cu(OH)2 .Cu(NO3)2
This is a multiple choice answer as classified in NCERT Exemplar
Correct option (ii)
When H2S is passed through an aqueous solution of salt acidified with dil. HCl in qualitative analysis, a black precipitate is produced. When the precipitate is boiled with dil. HNO3, a blue solution results. When an excess of ammonia aqueous solution is added to this solution, it produces a deep blue [Cu (NH3)4]2+ solution.
Commonly asked questions
Which of the following acids forms three series of salts?
(i) H3PO2
(ii) H3BO3
(iii) H3PO4
(iv) H3PO3
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii) H3PO4
When it reacts with a metal atom, it can produce three different forms of salts, referred known as the three series of salts.
H3PO4 + Na→NaH2PO4
NaH2PO4 + Na→Na2HPO4
Na2HPO4 + Na→Na3PO4
On heating ammonium dichromate and barium azide separately we get
(i) N2 in both cases
(ii) N2 with ammonium dichromate and NO with barium azide
(iii) N2O with ammonium dichromate and N2 with barium azide
(iv) N2O with ammonium dichromate and NO2 with barium azide
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i)
We get N2 in both situations when we heat ammonium dichromate and barium azide separately.
(NH4)2Cr2O7→Cr2O3 + 4H2O + N2
Ba (N3)2→Ba + 3N2.
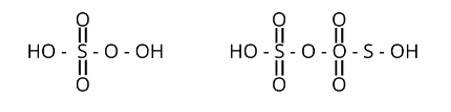
Which of the following are peroxoacids of sulphur?
(i) H2SO5 and H2S2O8
(ii) H2SO5 and H2S2O7
(iii) H2S2O7 and H2S2O8
(iv) H2S2O6 and H2S2O7
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i)
Peroxy linkage refers to the presence of a bond between oxygen and oxygen (O-O) in a molecule.
Only H2S2O6 and H2S2O7 of the sulphur oxoacids above have a peroxy linkage.
In the preparation of compounds of Xe, Bartlett had taken O2+ PtF6− as a base compound. This is because
(i) Both O2 and Xe have the same size.
(ii) Both O2 and Xe have the same electron gain enthalpy.
(iii) Both O2 and Xe have almost the same ionisation enthalpy.
(iv) Both O2 and Xe are gases.
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
Bartlett noticed that Xenon's first ionisation potential is nearly identical to that of oxygen and used Born-Haber calculations to predict the presence of a stable compound: Xe+ Pt F6-
Which of the following is an isoelectronic pair?
(i) ICl2 , ClO2
(ii) BrO2- , BrF2
(iii) ClO2 , BrF
(iv) CN- , O3
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (ii)
BrO2- (35+2×8+1=52) and BrF2+ (35+2×9−1=52)
52 electrons are present in both
Which of the following options are not in accordance with the property mentioned against them?
(i) F2 > Cl2 > Br2 > I2 Oxidising power.
(ii) MI > MBr > MCl > MF Ionic character of metal halide.
(iii) F2 > Cl2 > Br2 > I2 Bond dissociation enthalpy.
(iv) HI < HBr < HCl < HF Hydrogen-halogen bond strength.
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (ii), (iii)
MI > MBr > MCl > MF is an iconic character of metal halide
F2 > Cl2 > Br2 > I2 is a bond dissociation enthalpy
Which of the following statements are correct for SO2 gas?
(i) It acts as a bleaching agent in moist conditions.
(ii) It’s molecule has linear geometry.
(iii) It’s dilute solution is used as disinfectant.
(iv) It can be prepared by the reaction of dilute H2SO4 with metal sulphide.
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i) and (iii)
SO2 is employed as an antichlor, disinfectant, and preservative in the bleaching of wool and silk.
Which of the following orders are correct as per the properties mentioned against each?
(i) As2O3 < SiO2 < P2O3 < SO2 Acid strength.
(ii) AsH3 < PH3 < NH3 Enthalpy of vapourisation.
(iii) S< O < Cl < F More negative electron gain enthalpy.
(iv) H2O > H2S > H2Se > H2Te Thermal stability.
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i) and (iv)
(i) As2O3 < SiO2 < P2O3 < SO2 is the order of acid
(ii) AsH3 < PH3 < NH3 is the correct or enthalpy of vapourization
(iii) S < O < F < Cl is the correct order of more negative gain enthalpy
(iv) H2O > H2Se > H2Te is the order of thermal stability
Which of the following statements are correct?
(i) S–S bond is present in H2S2O6.
(ii) In peroxosulphuric acid H2SO4 sulphur is in +6 oxidation state.
(iii) Iron powder along with Al2O3 and K2O is used as a catalyst in the preparation of NH3 by Haber’s process.
(iv) Change in enthalpy is positive for the preparation of SO3 by catalytic oxidation of SO2.
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i)
Oxidation state of S=+6
(iii) Iron oxide with K2O and Al2O3 is used to increase the rate of attainment of equilibrium in Haber's process.
(iv) Change in enthalpy is negative for the preparation of SO3 by catalytic oxidation of SO2.
Which of the following statements are true?
(i) Only type of interactions between particles of noble gases are due to weak dispersion forces.
(ii) Ionisation enthalpy of molecular oxygen is very close to that of xenon.
(iii) Hydrolysis of XeF6 is a redox reaction.
(iv) Xenon fluorides are not reactive.
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i) and (ii)
(i) The modest dispersion force causes attraction in noble gases.
(ii) In nature, xenon fluorides are reactive.
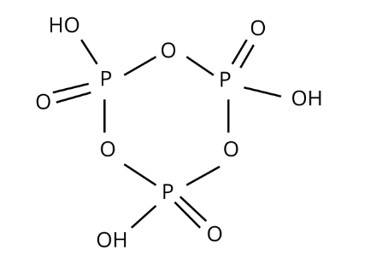
In a cyclotrimetaphosphoric acid molecule, how many single and double bonds are present?
(i) 3 double bonds; 9 single bonds
(ii) 6 double bonds; 6 single bonds
(iii) 3 double bonds; 12 single bonds
(iv) Zero double bonds; 12 single bonds
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
H3PO2, H3PO4, H3PO4, and other oxoacids are formed by phosphorus. P is tetrahedrally surrounded by other atoms in phosphorus oxoacids. At least one P-H bond and an O-H bond are known to form in all of them.
Which of the following elements can be involved in p\pi --d\pi bonding?
(i) Carbon
(ii) Nitrogen
(iii) Phosphorus
(iv) Boron
This is a multiple choice answer as classified in NCERT Exemplar
Correct option (iii)
If an electron pair is donated to a vacant orbital on one atom and a lone pair of electrons on the other, the bonding is designated pπ-dπ depending on the orbital to which the electron pair is donated and the orbital from which the electron pair is donated.
Because their valence shells lack d-orbitals, nitrogen, carbon, and boron cannot form a pi bond. Phosphorus, on the other hand, can produce pi bonds.
Which of the following pairs of ions are isoelectronic and isostructural?
(i) CO32- , NO3-
(ii) ClO3- , CO32-
(iii) SO32- , NO3-
(iv) ClO3- , SO32-
This is a multiple choice answer as classified in NCERT Exemplar
Correct option (i)
The compound has the following number of electrons:
NO3- = 32e-
CO32- = 32e-
ClO3- = 42e-
SO3 - = 42e-
Hence, NO3- and CO32- are isoelectronic
Affinity for hydrogen decreases in the group from fluorine to iodine. Which of the halogen acids should have highest bond dissociation enthalpy?
(i) HF
(ii) HCl
(iii) HBr
(iv) HI
This is a multiple choice answer as classified in NCERT Exemplar
Correct option (i)
Fluorine is the most reactive element, forming hydrogen fluoride when it combines with hydrogen gas.
Due to the shorter bond length between the two atoms, it takes a lot of energy to break the link between hydrogen and fluorine.
As a result, as we move down the group, the bond dissociation energy drops.
Bond dissociation enthalpy of E—H (E = element) bonds is given below. Which of the compounds will act as the strongest reducing agent?
Compound NH3 PH3 AsH3 SbH3
Δdiss(E---H)/kJ mol--1 389 322 297 255
(i) NH3
(ii) PH3
(iii) AsH3
(iv) SbH3
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iv)
The E-H bond dissociation energy of SbH3 is the lowest among the aforementioned compounds, it easily releases H, making it a strong reducing agent. The greater the lowering agent, the weaker the E-H bond.
On heating with concentrated NaOH solution in an inert atmosphere of CO2, white phosphorus gives a gas. Which of the following statements is incorrect about the gas?
(i) It is highly poisonous and smells like rotten fish.
(ii) It’s solution in water decomposes in the presence of light.
(iii) It is more basic than NH3.
(iv) It is less basic than NH3.
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
When heated with concentrated NaOH in an inert CO2 environment. It formsPH3
P4 + 3NaOH + 3H2O→PH3 + 3NaH2PO2
As we progress from the top to the bottom in a group, the basic character of hydrides reduces PH3 is basic than NH3.
Strong reducing behaviour of H3PO2 is due to
(i) Low oxidation state of phosphorus
(ii) Presence of two –OH groups and one P–H bond
(iii) Presence of one –OH group and two P–H bonds
(iv) High electron gain enthalpy of phosphorus
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
H3PO2 has one O-H group and two P-H bonds
The existence of a P-H link gives phosphorus oxyacids their reducing characteristics. It has a strong proclivity for releasing protons. As a result, it demonstrates a decreasing nature.
On heating lead nitrate forms oxides of nitrogen and lead. The oxides formed are ______.
(i) N2O, PbO
(ii) NO2, PbO
(iii) NO, PbO
(iv) NO, PbO2
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (ii)
On heating, lead nitrate decomposes into lead monoxide, nitrogen dioxide, and oxygen.
2Pb (NO3)2→2PbO + 4NO2 + O2.
Which of the following elements does not show allotropy?
(i) Nitrogen
(ii) Bismuth
(iii) Antimony
(iv) Arsenic
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i)
The single N-N bond is weak because of high interelectronic repulsion of the non-bonding electrons, owing to the small bond length. As a result the catenation tendency is weaker in nitrogen that is why it does not show allotropy
Maximum covalency of nitrogen is ______________.
(i) 3
(ii) 5
(iii) 4
(iv) 6
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (ii)
The valence electrons of nitrogen are two 2s and three 2p. Nitrogen can make three covalent bonds by sharing its three 2p electrons. Consider the NH3 molecule. The nitrogen atom, however, still contains a lone pair of electrons in the 2s orbital. It can take one bond by transferring these two electrons from the lone pair. Consider the NH4+ molecule.
As a result, nitrogen can create four different bonds.
Which of the following statements is wrong?
(i) Single N–N bond is stronger than the single P–P bond.
(ii) PH3 can act as a ligand in the formation of coordination compound with transition elements.
(iii) NO2 is paramagnetic in nature.
(iv) Covalency of nitrogen in N2O5 is four
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i)
The N-N sigma bond (single bond) is weaker than the P-P sigma bond (single bond) due to the smaller size of nitrogen which increases the interelectronic repulsions and thus making the bond weaker.
A brown ring is formed in the ring test for NO3- ion. It is due to the formation of
(i) [Fe(H2O)5(NO)]2+
(ii) FeSO4 .NO2
(iii) [Fe(H2O)4(NO)2]2+
(iv) FeSO4.HNO3
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i)
The 'Brown Ring Test' is an identification and conformation test of the nitrate ion (NO3- ) using conc. H2SO4 and newly produced FeSO4. Place the NO3- containing sample in a test tube. Then, with gentle shaking, add conc H2SO4 dropwise, heat it briefly on a Bunsen burner, and add freshly made greenish FeSO4 Then, due to the creation of brown coloured [Fe (H2O)5 (NO)]SO4 complex, a brown ring forms in that test tube.
Elements of group-15 form compounds in +5 oxidation state. However, bismuth forms only one well characterised compound in +5 oxidation state. The compound is
(i) Bi2O5
(ii) BiF5
(iii) BiCl5
(iv) Bi2S5
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (ii)
Bismuth is the sole element that has an inert pair effect. Only generates trihalides and has a +3 oxidation state. Florine, on the other hand, is small and has a high electronegativity.
In the preparation of HNO3, we get NO gas by catalytic oxidation of ammonia. The moles of NO produced by the oxidation of two moles of NH3 will be______.
(i) 2
(ii) 3
(iii) 4
(iv) 6
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i)
The catalytic oxidation of ammonia produces NO gas, which is used to make HNO3. 4 moles of NH3 created 4 moles of NO in the equation below. As a result, the moles of NO produced by oxidising two moles of NH3 will be two moles.
4NH3 + 5O2→4NO + 6H2O.
The oxidation state of central atom in the anion of compound NaH2PO2 will be ______.
(i) +3
(ii) +5
(iii) +1
(iv) –3
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
Na (H2)PO2
1+ (2x+1)+ x +2 (−2)=0
x−1=0
x=1
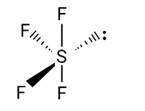
Which of the following is not tetrahedral in shape?
(i) NH4+
(ii) SiCl4
(iii) SF4
(iv) SO42−
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
The C-atom in the CO32- ion undergoes sp2 hybridization. BF4, NH4+ and SO42- have a tetrahedral structure, whereas it has a triangular planar structure.
Hot conc. H2SO4 acts as moderately strong oxidising agent. It oxidises both metals and nonmetals. Which of the following element is oxidised by conc. H2SO4 into two gaseous products?
(i) Cu
(ii) S
(iii) C
(iv) Zn
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
C+2H2SO4→CO2+2SO2+2H2O
Conc. H2SO4 oxidises C to produce two gaseous products.
A black compound of manganese reacts with a halogen acid to give greenish yellow gas. When excess of this gas reacts with NH3 an unstable trihalide is formed. In this process the oxidation state of nitrogen changes from _________.
(i) – 3 to +3
(ii) – 3 to 0
(iii) – 3 to +5
(iv) 0 to – 3
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iv)
MnO2 ( Black )+4HCl→MnCl2+2H2O+Cl2
Greenish yellow color
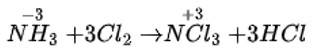
In solid state PCl5 is a _________.
(i) Covalent solid
(ii) Octahedral structure
(iii) Ionic solid with [PCl6]+ octahedral and [PCl4]− tetrahedral
(iv) Ionic solid with [PCl4]+ tetrahedral and [PCl6]− octahedral
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iv)
The ionic bonding promotes the crystalline structure of PCl5 in the solid state by attempting to exist as oppositely charged ions like [PCl4]+ and [PCl6]− . Also, [PCl4]+ and [PCl6]− are tetrahedral and octahedral, respectively. These structures fit together well, giving the solid structure extra stability.
Reduction potentials of some ions are given below. Arrange them in decreasing order of oxidising power.

(i) ClO4- > IO4- > BrO4-
(ii) IO4- > BrO4- > ClO4-
(iii) BrO4- > IO4- > ClO4-
(iv) BrO4- > ClO4- > IO4-
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
The ability of a substance to be decreased is known as its reduction potential. As the substance's reduction potential rises, so does its power to reduce. It signifies the chemical's oxidising power (the ability of the material to cause other substances to lose electrons, which is known as oxidising power) grows.
As a result, the oxidising power is listed in decreasing order BrO4- > IO4- > ClO4-
If chlorine gas is passed through hot NaOH solution, two changes are observed in the oxidation number of chlorine during the reaction. These are ________ and _________.
(i) 0 to +5
(ii) 0 to +3
(iii) 0 to –1
(iv) 0 to +1
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i) and (iii)
6NaOH+3Cl2 → 5NaCl+NaClO3+3H2O
Chlorine gas oxidation number ranges from 0 to –1 to 0 to +5.
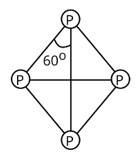
Which of the following is correct for P4 molecule of white phosphorus?
(i) It has 6 lone pairs of electrons.
(ii) It has six P–P single bonds.
(iii) It has three P–P single bonds.
(iv) It has four lone pairs of electrons.
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (ii) and (iv)
Tetrahedral geometry characterises the structure of a white phosphorus molecule.
Phosphorus has a valency of 5, and there are four phosphorus atoms in total. As a result, there will be a total of 4×5=20 valence electrons.
As a result, each P atom will have one lone pair of electrons, and each covalent bond (two electrons) will be established.
In a molecule of white phosphorus, there will be four lone pairs of electrons and six P-P single bonds in total.
Which of the following statements are correct?
(i) Among halogens, the radius ratio between iodine and fluorine is maximum.
(ii) Leaving the F—F bond, all halogens have weaker X—X bonds than X—X'bonds in interhalogens.
(iii) Among interhalogen compounds maximum number of atoms are present in iodine fluoride.
(iv) Interhalogen compounds are more reactive than halogen compounds.
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i)
Due to its high electronegativity and tiny size, F is the only interhalogen with weaker X-X’ bonds and no X-X bonds.
Which of the following statements are correct?
(i) All the three N—O bond lengths in HNO3 are equal.
(ii) All P—Cl bond lengths in PCl5 molecule in gaseous state are equal.
(iii) P4 molecule in white phosphorus have angular strain therefore white phosphorus is very reactive.
(iv) PCl is ionic in solid state in which cation is tetrahedral and anion is octahedral.
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii) and (iv)
The P4 molecule in white phosphorus has an angular strain, white phosphorus is very reactive.
PCl5 is ionic in the solid state, with a tetrahedral cation and an octahedral anion.
In which of the following reactions conc. H2SO4 is used as an oxidising reagent?
(i) CaF2 + H2SO4 →CaSO4 + 2HF
(ii) 2HI + H2SO4 → I2 + SO2 + 2H2O
(iii) Cu + 2H2SO4 →CuSO4 + SO2 + 2H2O
(iv) NaCl + H2SO4 → NaHSO4 + HCl
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (ii) and (iii)
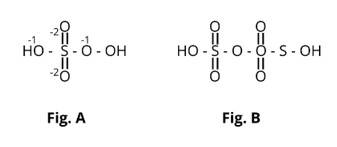
Here fig (b) contains one S-S bond.
The oxidising behaviour of H2SO4 is represented by (ii) and (iii) among the aforementioned four. It oxidises HI in reaction (ii) and then reduces to SO2.
The oxidation state of sulphur's core atom drops from +6 to +4. It oxidises copper in (iii) and is reduced to SO2.
26th February 2021 (Second Shift)
26th February 2021 (Second Shift)
Commonly asked questions
Match List – I with List – II.
List – I List – II
(A) Siderite (i) Cu
(B) Calamine (ii) Ca
(C) Malachite (iii) Fe
(D) Cryolite (iv) Al
(v) Zn
Choose the correct answer from the options given below
Siderite – FeCO3 (ore of iron)
Calamine – ZnCO3 (ore of zinc)
Malachite – CuCO3.Cu (OH)2 (ore of copper)
Cryolite – Na3AlF6 (ore of aluminium)
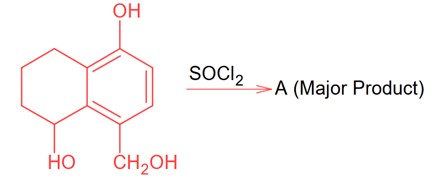
Identify A in the given reaction.
Since SOCl2 is used to covert aliphatic (R-OH) into chlorides. It will not react with aromatic alcohol
Which of the following forms of hydrogen emits low energy β - particles?
Tritium is radioactive and it decays into He3 during emission of β-radiation
1T3 → 2He3 + -1e0
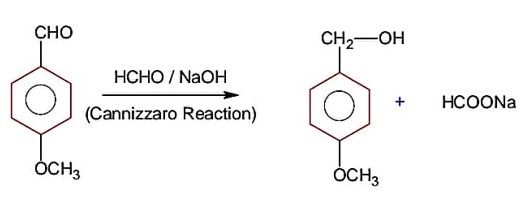
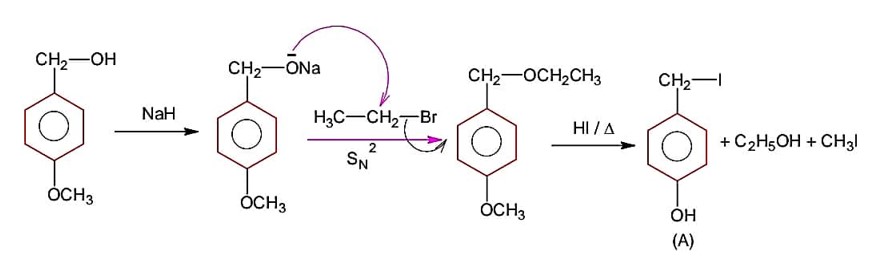
Identify A in the following chemical reaction.
The nature of charge on resulting colloidal particles when FeCl3 is added to excess of hot water is
Match List – I and List – II.
Choose the correct answer from the options given below
(c)
(d)
Ceric ammonium nitrate and CHCl3/alc. KOH are used for the identification of functional groups present in…………. and……………respectively.
Ceric ammonium nitrate is used to test alcohol while CHCl3/alc. KOH is used to test 1° amine
Calgon is used for water treatment. Which of the following statement is NOT true about Calgon?
It does not contains 2nd most abundant element by weight in earth crust because that is Si Calgon
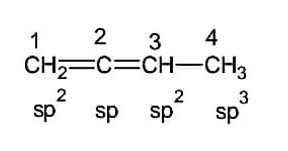
In molecule, the hybridization of carbon 1,2,3 and 4 respectively are
Consider the following image
Match List-I with List-II.
List – I List – II
(A) Sucrose (i) β-D-Galactose and β-D-Glucose
(B) Lactose (ii) α-D-Glucose and β-D-Fructose
(C) Maltose (iii) α-D-Glucose and α-D-Glucose
Choose the correct answer from the options given below:
(a) Sucrose → α-D glucose and β-D fructose
(b) Lactose → β-D- galactose and β-D glucose
(c) maltose → α-D glucose and α-D-glucose
A 2.0g sample containing MnO2 is treated with HCl liberating Cl2. The Cl2 gas is passed into a solution of Kl and 60.0mL of 0.1M Na2S2O3 is required to titrate the liberated iodine. The percentage of MnO2 in the sample is_______. (Nearest integer)
[Atomic masses (in u) Mn = 55; Cl = 35.5; O = 16, I = 127, Na = 23, K = 39, S = 32]
Here, meq of MnO2 = meq of Na2S4O6
Mass of MnO2 in sample = 0.261 g
Percentage of MnO2 in sample =
= 13.05%
If the work function of a metal is 6.63 × 10-19J, the maximum wavelength of the photon required to remove a photoelectron from the metal is _______nm. (Nearest integer)
[Given h = 6.63 × 10-34 Js, and c = 3 × 108 ms-1]
Work function,
Threshold wavelength
Using ;
=300 × 10-9 m = 300 nm
The hybridization of P exhibited in PF5 is spxdy. The value of y is_________
Hybridization of P in PF5 is sp3d, so value of y = 1
4.0 L of an ideal gas is allowed to expand isothermally into vacuum until the total volume is 20L. The amount of heat absorbed in this expansion is _________L atm.
For expansion in vacuum, workdone, w = 0
For isothermal process,
According to first law of thermodynamics,
The vapour pressure of two volatile liquid A and B at 25°C are 50 Torr and 100 Torr, respectively. If the liquid mixture contains 0.3 mole fraction of A, then the mole fraction of liquid B in the vapour phase is The value of x is____________.
Mole fraction of A in liquid phase, xA = 0.3
Mole fraction of B in liquid phase, xB = 0.7
Now;
= 100 × 0.7 = 70 torr
Mole fraction of B in vapour phase,
X = 14
The solubility product of a sparingly soluble salt A2X3 is 1.1 × 10-23. If specific conductance of the solution is 3 × 10-1, the limiting molar conductivity of the solution is x × 10-3 S m2 mol-1. The value of x is_________.
Using ; ksp = 108 S5
1.1 × 10-23 = 108 S5
S =
Specific conductance,
= 3 × 10-3 Sm2 mol-1
So; x = 3
The quantity of electricity in Faraday needed to reduce 1 mol of to Cr3+ is________.
Here, 6F electricity is required to reduce 1 mol to Cr3+.
For a first order reaction A®B, the rate constant, k = 5.5 × 10-14s-1. The time required for 67% completion of reaction is x × 10-1 times the half life of reaction. The value of x is ________ (Nearest integer)
(Given : log 3 = 0.4771)
Rate constant, k = 5.5 × 10-14 s-1.
=
From (i) & (ii)
= 1.58 t50%
So; t67% is 15.8 × 10-1 times half life.
X = 16 (the nearest integer)
Number of complexes which will exhibit synergic bonding amongst and is_______________.
All metal carbonyls have synergic bonds
In the estimation of bromine, 0.5g of an organic compound gave 0.40g of silver bromide. The percentage of bromine in the given compound is____________% (Nearest integer)
(Relative atomic masses of Ag and Br are 18 u and 80 u, respectively).
Organic compound → AgBr (s)
0.5 g 0.40 g
Here; Moles of Br in organic compound = Moles of Br in Ag Br
= Moles of AgBr
=
Mass of Br in organic compound =
Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven Exam