Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven
Get insights from 115 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
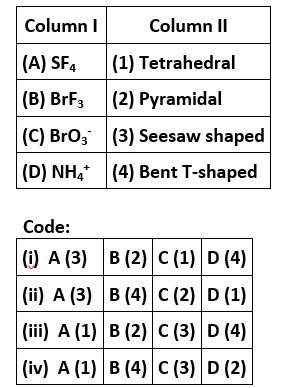
New answer posted
6 months agoContributor-Level 10
This is a matching answer type question as classified in NCERT Exemplar
Correct option is (iii)
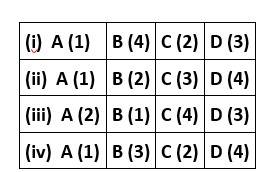
New answer posted
6 months agoContributor-Level 10
This is a matching answer type question as classified in NCERT Exemplar
Correct option is (ii)
New answer posted
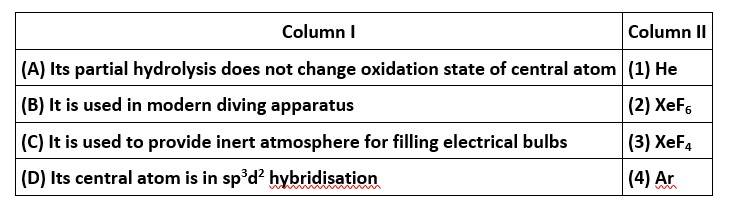
6 months agoContributor-Level 10
This is a matching answer type question as classified in NCERT Exemplar
Correct option is (i)
CCl3NO2 stands for chloropicrin, which is known as tear gas. Cl is the element of the highest electron gain enthalpy. Sulphur is the element belonging to 16th group, also known as chalcogens.
New answer posted
6 months agoContributor-Level 10
This is a matching answer type question as classified in NCERT Exemplar
Correct option is (ii)
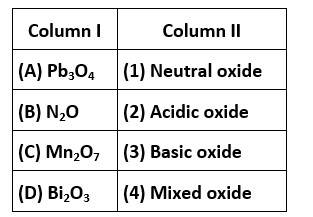
A. Pb3O4is a mixed ore
B. N2O is a neutral oxide
C. Mn2O7is acidic oxide
D. Bi2O3is basic oxide
New answer posted
6 months agoContributor-Level 10
This is a matching answer type question as classified in NCERT Exemplar
Correct option is (i)
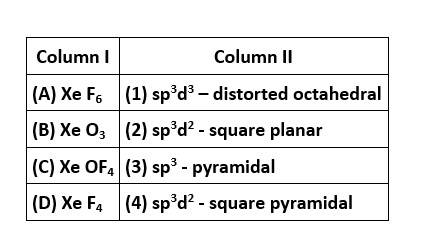
In XeF6, the central Xe atom undergoes sp3d3 hybridisation and the molecular geometry is distorted octahedral with 1 lone pair and 6 bond pairs of electrons.
In XeO3, the central Xe atom undergoes sp3 hybridisation and the molecular geometry is pyramidal with 1 lone pair and 3 bonding domains of electrons.
In XeOF4, the central Xe atom undergoes sp3d2 hybridization and the molecular geometry is square pyramidal with 1 lone pair and 5 bond pairs of electrons.
In XeF4, the central Xe atom undergoes&nb
New answer posted
6 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
Correct option is (i)Here, the S atom is surrounded by 6F atoms, so it's complected for H2O to attack SF6, but in case of SF4, it's surrounded by 4F atoms, so it's to attack H2O.
New answer posted
6 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
Correct option is (i)
When NaCl reacts with concentrated H2SO4, it produces colourless fumes with a pungent smell, but when MnO2 is added, the fumes turn greenish yellow. MnO2 oxidizes HCl to chlorine gas, which then turns greenish yellow.
New answer posted
6 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
Correct option is (i)
The rhombic and the monoclinic forms of sulphur exist as S8 . Oxygen forms a p\pi - p\pi multiple bond due to its small size and short bond length, but Pπ- Pπ bonding is not possible because of sulphur's larger atomic size.
New answer posted
6 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
Correct option is (ii)
Because H2SO4 is a stronger oxidising agent and HI is a stronger reducing agent, H2SO4 oxidizes and is reduced to SO2.
New answer posted
6 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
Correct option is (iii)
HNO3 forms an oxide layer on the surface of iron. This is also known as corrosion or rusting.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers