Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven
Get insights from 115 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
0.001 M NaOH solution has [OH-] = 0.001 M = 10-3 M
Using ; pOH =
=
pOH = 3
pH = 14 – pOH
pH = 14 – 3
pH = 11
New answer posted
6 months agoContributor-Level 10
Fluorine forms only one oxoacid which is hypofluorous acid HOF because it shows only – 1 oxidation state. Which is due to its the smallest size among halogens & the highest electronegativity.
New answer posted
6 months agoContributor-Level 10
Structure PCl5 is trigonal bipyramidal,
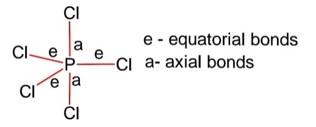
Hybridization of P is sp3d
Equatorial bonds lie in a plane
Axial bonds are longer than equatorial bonds so axial bonds are weaker than equatorial bonds.
New answer posted
6 months agoContributor-Level 10
Leaching involves the given reaction,
Here, O2 is required for formation of Au (l) cyanide complex but no complex in absence of O2.
In above displacement reaction, Zn is oxidized.
New answer posted
6 months agoContributor-Level 10
The equation can be written as-
Ap+x + Bq- y ? xAp+ (aq) + yBq-
S moles of A and B dissolves to give x S moles of Ap+ and y S moles of Bq-.
Ksp = [Ap+]x [Bq-]y = [x5]x [y5]y
= xx y y 5 x+y
New answer posted
6 months agoContributor-Level 10
Xenon form compounds due to the high electronegativity of fluorine and oxygen, low ionization energy compared to lighter noble gases, and the availability of empty d-orbitals. This breaks the old assumption about noble gases being entirely inert.
New answer posted
6 months agoContributor-Level 10
With the combination of two different halogens, the interhalogen compounds are formed. When compared with pure diatomic halogen molecules, they are more reactive because the bond between different halogens are more polar and weaker. During reactions, it makes them more susceptible to bond cleavage.
New answer posted
6 months agoContributor-Level 10
Due to their high electronegativity, small atomic size, greater ability to form multiple bonds, and absence of d-orbitals, the first elements of each group behave differently.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers