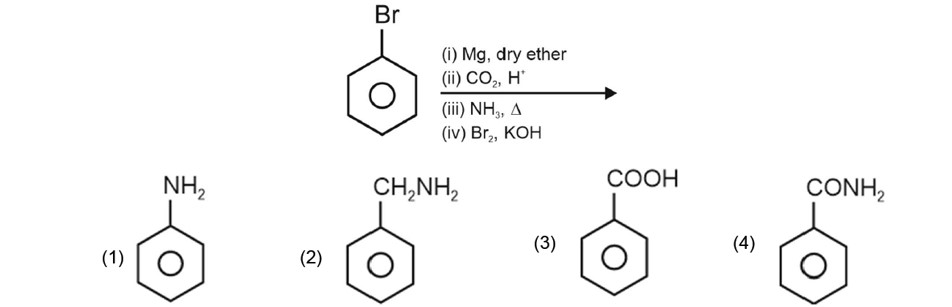
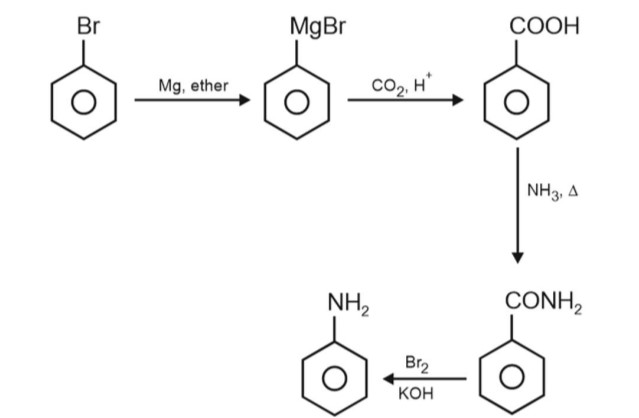
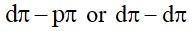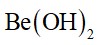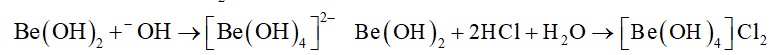Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Each of the element in group III
Mn is in group seven shows a maximum oxidation state of +7
New answer posted
4 months agoContributor-Level 10
Group?17, elements have very high ionisation enthalpy due to increase in atomic size, ionization enthalpy decreases down the group.
New answer posted
4 months agoContributor-Level 10
In the metallurgy of aluminium, purified Al2O3 is mixed with Na3AIF6 or CaF2 which lowers the melting point of the mixture and brings conductivity.
New answer posted
4 months agoContributor-Level 10
Sucrose is non-reducing sugar. It does not reduce Fehling solution.
New answer posted
4 months agoContributor-Level 10
Heavier element of p block do not from pπ− pπ bonds as their atomic orbital are so large and diffius that they cannot have effecitve overlapping.
New answer posted
4 months agoContributor-Level 10
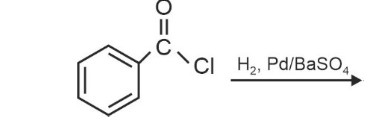
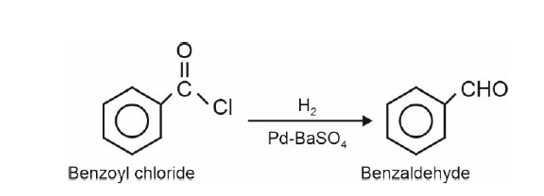
Acyl chloride is hydrogenated over catalyst, palladium or barium sulphate. This reaction is called Rosenmund reaction.
New answer posted
4 months agoContributor-Level 10
The inertness of
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers