Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
B2 have bond order equal to 1 and also paramagnetic.
have bond order equal to 1 but are diamagnetic.
have bond order equal to 3.
New answer posted
4 months agoContributor-Level 10
After the 2023-24 revised CBSE syllabus, many chapters have been removed, and in some chapters syllabus is reduced. As per the latest CBSE exam pattern 2025-26, the Chapter 1 Solutions will be asked for 5-7 marks in the Class 12 Chemistry CBSE board exams.
For other competitive exams, the Solutions chapter has a medium weightage in the NEET exam. In the NEET exam, 1-2 questions are frequently asked based on the concept of moderate-level numerical.
Physical Chemistry holds over 30% weightage in the chemistry section in the JEE Mains exam. The solutions chapter is considered a part of physical chemistry. You can expect 1 question fo
New answer posted
4 months agoContributor-Level 10
Fehling solution test can be given by aldehyde except aromatic aldehyde
New answer posted
4 months agoContributor-Level 10
Assume mass of solution
= 100g
Mass of solute = 36gm
Moles of solute = 1
Molarity=
New answer posted
4 months agoContributor-Level 10
37Rb = 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s1
Last electron enters in 5s subshell
Value of quantum numbers
n = 5, l = 0, m = 0, s =
New answer posted
4 months agoBeginner-Level 5
NCERT offers the foundation for every class 12 student. CBSE board exam questions are primarily based on the NCERT exercise, Examples and conceptual explanation.
There are various benefits of using NCERT Solutions.
- Specially for CBSE Board students, these NCERT Solutions are the best resource to prepare for the boards.
- These NCERT Solutions include step-wise descriptive answer approach that will help you write good answers.
- These solutions also help you understand problem-solving and how to approach a specific type of conceptual or numerical problem.
- If you are preparing for the JEE or NEEt exam, these solutions can be your first deci
New answer posted
4 months agoContributor-Level 10
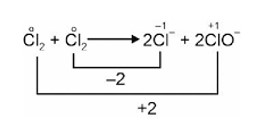
After balancing change in oxidation state,
2Cl2 2Cl– + 2ClO–
Next, balance 'O' atoms,
2Cl2 +4OH–
Simplifying to get the simplest ratios,
Cl2 +2OH–
x = 1, y = 2, z = 1, p = 1
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers