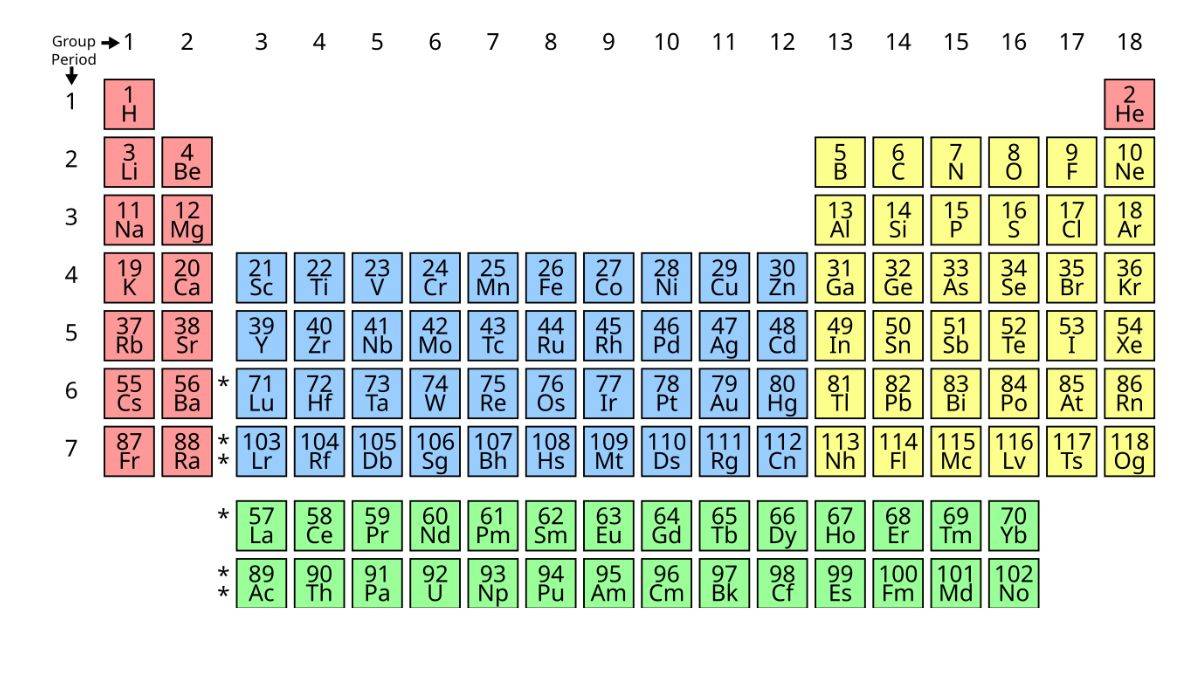
The periodic table, also known as the map of chemistry, brings order to a subject and simplifies the periodic classification of elements. With more than 100 elements known today, learning their properties one by one would be almost impossible. The periodic table groups elements with similar behavior together, making it easier to understand their chemistry and even predict the nature of elements that are yet to be discovered.
Below you’ll find the class 11 chemistry ch3 notes covering all the topics, such as:
- Why Do We Need To Classify Elements?
- Genesis Of Periodic Classification
- Modern Periodic Law And The Present Form Of The Periodic Table
- Nomenclature Of Elements With Atomic Numbers > 100
- Electronic Configurations Of Elements And The Periodic Table
- Electronic Configurations And Types Of Elements: S-, P-, D-, F- Blocks
- Periodic Trends In Properties Of Elements
To practice the exercise questions, you can also check Class 11 Chemistry Ch3 NCERT solutions. The notes below are created to save your time and prepare each topic within a short time. With all the important topics of the periodic classification of elements class 11, you can prepare for your board exam and practice for the JEE and NEET exams.
- Classification of Periodic Elements
- Genesis of Periodic Classification
- The Modern Periodic Table
- Nomenclature of Elements with Atomic Numbers > 100
- Electronic Configurations Of Elements And The Periodic Table
- Electronic Configurations and Types Of Elements: s-, p-, d-, f- Blocks
- Periodic Trends in Properties of Elements
- Revision Notes for Class 11 Chemistry
- NCERT Solutions for Class 11 Chemistry
- About the Content Reviewer
- Classification of Elements and Periodicity in Properties FAQs
Classification of Periodic Elements
In the early 19th century, only 31 elements had been identified. By the mid-1800s, this number doubled, and today we know 118 elements. But you all may have a question- why do we need to classify elements? Well, each element has unique features, but many show similarities.
Why Do We Need to Classify Elements?
Arranging elements into groups helps in:
- Easy to remember properties
- Can guess properties of new elements
- See patterns in how elements behave
- Makes chemistry simpler to study
For example, Mendeleev left a space for Gallium in his table and described it as “Eka-Aluminium.” When Gallium was actually discovered later, it matched his predictions almost exactly.
Genesis of Periodic Classification
Dobereiner’s Method
A German scientist named Dobereiner found that some elements could be put in groups of three and called them triads.
What he found is that:
- Three elements in each group had similar properties
- The middle element’s weight was half of the sum of the other two
Examples:
- Lithium (Li)- 7, Sodium (Na)-23, Potassium (K)- 39
- Chlorine (Cl)-35.5, Bromine (Br)-80, Iodine (I)-127
But there are some limitations:
Limitations of Dobereiner triads-
- This worked for only a few elements, not all.
Newland’s Law of Octaves
An English scientist, Newland, arranged elements by their weight. He found that every 8th element was similar to the first one, like musical notes!
Example:
Li, Be, B, C, N, O, F | Na (similar to Li), Mg (similar to Be)…
But with this pattern, the limitations of Newland’s Octaves are that it breaks down after the 20th element.
Mendeleev’s Periodic Table
A Russian scientist named Mendeleev made the best arrangement at that time.
What he did was arrange elements by increasing weight, put similar elements in the same column, leave empty spaces for elements not yet found, and sometimes change the order to keep similar elements together.
Mendeleev’s Law, or periodic law, states that “Element properties are periodic functions of their atomic weight.”
The advantages or success of Mendeleev’s periodic table were that he predicted properties of missing elements, and when they were found later, his prediction was correct. He corrected the wrong atomic masses of some elements, and he left space for elements that had not yet been discovered, such as Gallium.
The limitations of Mendeleev’s law are:
- Where to put hydrogen? Because it was in the group of alkali metals, but also has the properties of a halogen.
- No explanation for isotopes, because classification of elements is done based on atomic weight, so protium, deuterium, and tritium would take different positions.
- Some elements, like cobalt and nickel, are placed oddly.
- Couldn’t explain why properties repeated
The Modern Periodic Table
A scientist named Moseley discovered that the atomic number (number of protons) is more important than atomic weight.
The Modern Periodic Table states that “Element properties repeat regularly when arranged by atomic number.”
This solved all the problems with Mendeleev’s table. Here how the periodic table NCERT looks like:
Here is the detailed breakdown of the periodic table class 11 chapter 3:
- 18 columns called Groups (numbered 1-18)
- 7 rows called Periods (numbered 1-7)
- 118 elements are known so far
In Groups :
- Elements have the same number of outer electrons
- They behave similarly
- Example: Group 1 has Li, Na, K, Rb, Cs - all react with water
In Periods:
- Elements have the same number of electron shells
- Properties change as you move from left to right
- Example: Period 2 has Li (metal) to Ne (gas)
Special Groups:
- Group 1: Alkali metals (very reactive)
- Group 2: Alkaline earth metals
- Group 17: Halogens (very reactive non-metals)
- Group 18: Noble gases (don’t react easily)
Nomenclature of Elements with Atomic Numbers > 100
When scientists make new heavy elements (atomic number > 100), they give them temporary names using numbers.
Number words used:
- 0 = nil, 1 = un, 2 = bi, 3 = tri, 4 = quad, 5 = pent
- 6 = hex, 7 = sept, 8 = oct, 9 = enn
Examples:
- Element 104: Un-nil-quad-ium (now called Rutherfordium)
- Element 118: Un-oct-oct-ium (now called Oganesson)
Electronic Configurations Of Elements And The Periodic Table
Electron blocks (Orbitals)- Electrons living in different blocks around the nucleus. These blocks are called orbitals.
Types of blocks:
- s-block: Round shape, holds 2 electrons
- p-block: Dumbbell shape, holds 6 electrons
- d-block: Complex shape, holds 10 electrons
- f-block: Very complex shape, holds 14 electrons
Filling Order
Electrons fill orbitals in order of energy - lowest energy first.
Order: 1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p…
Connection to Periodic Table
- Period number = Number of electron shells
- Group number = Number of outer electrons (for main groups)
Examples:
- Sodium (Na): 3 shells, so it’s in Period 3
- Sodium has 1 outer electron, so it’s in Group 1
Electronic Configurations and Types Of Elements: s-, p-, d-, f- Blocks
In chemistry, Elements are divided into their particular group based on their characteristics and general configuration. You can check the electronic configurations and types of elements divided into each group below:
| Block |
Groups |
General Configuration |
Examples |
Characteristics |
| s-block |
1, 2 |
ns¹–ns² |
Na, Mg, Ca |
Soft, highly reactive metals |
| p-block |
13–18 |
ns²np¹–ns²np⁶ |
B, O, Cl, Ne |
Includes metals, non-metals, and noble gases |
| d-block |
3–12 |
(n-1)d¹–¹⁰ ns⁰–² |
Fe, Cu, Zn |
Transition metals, coloured compounds |
| f-block |
Lanthanoids & Actinoids |
(n-2)f¹–¹⁴ |
Ce, U |
Inner transition metals, radioactive |
s-Block Elements (Groups 1-2)
Last electron goes in: s-orbital
Examples: Li, Na, K (Group 1); Be, Mg, Ca (Group 2)
Properties of s-block elements:
- Metals
- React easily
- Give away electrons easily
- Shiny when cut
- Conduct electricity
p-Block Elements (Groups 13-18)
Last electron goes in: p-orbital
Examples: Al, C, N, O, F, Ne
Properties of p-block elements:
- Mix of metals, non-metals, and in-between elements
- Very different properties
- Some conduct, some don’t
d-Block Elements (Groups 3-12)
Last electron goes in: d-orbital
Examples: Fe, Cu, Zn, Ag, Au
Properties of d-block elements:
- All metals
- Can have different charges
- Often colored
- Good catalysts
- High melting points
f-Block Elements
Bottom two rows, last electron goes in: f-orbital
Two groups:
- Lanthanoids (top row)
- Actinoids (bottom row)
Properties of f-block elements:
- All metals
- Very similar to each other
- Many are radioactive
Periodic Trends in Properties of Elements
According to the periodic classification of elements, class 11, the properties of elements are related to the electronic configuration of their atoms. Below, we have discussed periodic trends in properties of elements based on atomic size, ion size, electron gain enthalpy, electronegativity, ionization energy, and metallic character.
Atomic Radius
Basically, how big an atom is.
Across a period (left to right) atom size gets smaller because more protons pull electrons closer. Whereas, down a group (top to bottom) atom size gets bigger because more electron shells are added.
Examples:
- Period 2: Li > Be > B > C (getting smaller)
- Group 1: Li < Na < K < Rb (getting bigger)
Ionic Radius
How big an atom becomes when it gains or loses electrons.
- Positive ions (lost electrons): Smaller than the original atom
- Negative ions (gained electrons): Bigger than the original atom
Examples:
- Na⁺ is smaller than Na
- Cl⁻ is bigger than Cl
Ionization Enthalpy
Ionization energy is the energy needed to remove an electron from an atom.
- Across a period: Generally increases because stronger pull from more protons
- Down a group: Decreases because electrons are farther away, easier to remove
Some exceptions to the ionization energy:
- Boron < Beryllium (different orbital types)
- Oxygen < Nitrogen (electron pairing)
Examples:
- Li < Be < B < C < N < F (mostly increasing)
- Li > Na > K > Rb (decreasing down group)
Electron Gain Enthalpy
It is the Energy change when an atom gains an electron.
- Across a period: Generally increases (becomes more negative)
- Down a group: Generally decreases (becomes less negative)
Special cases:
- Noble gases don’t want extra electrons
- Fluorine is less than chlorine (too small, electrons repel)
Electronegativity
How much an atom wants electrons in a bond is known as electronegativity.
- Across a period: Increases
- Down a group: Decreases
- Highest: Fluorine (4.0)
- Lowest: Cesium (0.7)
Uses of electronegativity:
- Predict if a bond will be ionic or covalent
- Understand molecule shapes
Metallic Character
How much an element behaves like a metal shows its metallic character.
- Across a period: Decreases (metals → non-metals)
- Down a group: Increases
Period 3 example: Na (metal) → Mg (metal) → Al (metal) → Si (metalloid) → P (non-metal) → S (non-metal) → Cl (non-metal)
Revision Notes for Class 11 Chemistry
NCERT Solutions for Class 11 Chemistry
About the Content Reviewer
Classification of Elements and Periodicity in Properties FAQs
Commonly asked questions
Why is the Classification of Elements Necessary?
Classification of Elements is Necessary because it helps in understanding and remembering elements' properties better, you can understand how elements react or behave, and can guess properties of unidentified or newly discovered elements.
What is meant by periodicity in the properties of elements?
When the elements having similar properties repeat at regular intervals, it is called periodicity in the properties of elements.
How to study the classification of elements and periodicity in properties?
You can read the classification of elements and periodicity in properties class 11 notes to study class 11 ch3 and score well in class 11th exams and NEET, and JEE exams.
Chemistry Classification of Elements and Periodicity in Properties Exam
Student Forum
Other Topics under this Chapter
- Classification of Elements
- Electronic Configuration Types of Elements
- Why do we need to classify elements
- Periodic Trends in Properties of Elements
- Genesis of Periodic Classification
- Present Form of Periodic Table
- Nomenclature of Elements with Atomic Numbers
- Periodic Table Electronic Configuration of Element
Other Class 11th Chemistry Chapters
- Chemistry Chemical Equilibrium
- Chemistry Structure of Atom
- Chemistry Redox Reactions
- Chemistry Some Basic Concepts of Chemistry
- Chemistry Organic Chemistry
- NCERT Class 11 Chemistry
- Chemistry Classification of Elements and Periodicity in Properties
- Chemistry Chemical Bonding and Molecular Structure
- Chemistry Hydrocarbon
- Chemistry Thermodynamics