Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
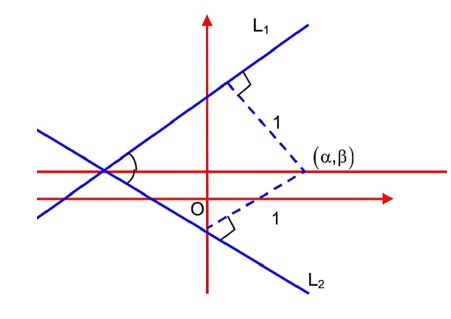
L1 : 3x – 4y + 12 = 0
L2 : 8x + 6y + 11 = 0
lies on that angle which contain origin
Equation of angle bisector of that angle which contain origin is
lies on it
…… (i)
……. (ii)
Solving (i) & (ii)
New answer posted
5 months agoContributor-Level 10
(a) Polluted water has low value of dissolved oxygen, but high value of B.O.D., because chemical and organic matter uses dissolved oxygen to decompose.
(b) Eutrophication is result of excessive growth of weed in water bodies, which consumes dissolved oxygen, thus decreases oxygen.
New answer posted
5 months agoContributor-Level 10
Cu is least reactive metal and it lies below hydrogen in reactivity series.
New answer posted
5 months agoContributor-Level 10
(a) low solubility of LiF in H2O due to high lattice energy.
(b)
(c) Na in liq NH3 forms electronated ammonia thus conducting in nature.
(d)
New answer posted
5 months agoContributor-Level 10
In clark's method Ca (OH)2 is used for softening hard water.
Thus CaCO3 and Mg (OH)2 are formed in reaction.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers