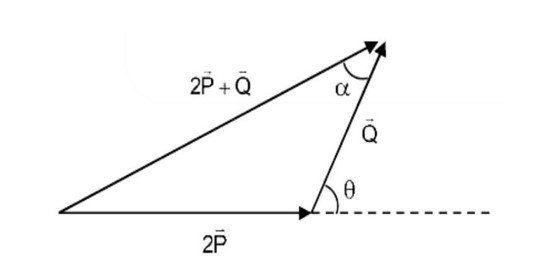
Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
5 months agoNew answer posted
5 months agoContributor-Level 10
Yes. Escape speed from a planet or celestial body is the same for all objects. It is irrespective of their mass. This is because the gravitational force and potential energy depend on the mass of the celestial body and distance. The object's own mass cancels out when deriving escape speed.
New answer posted
5 months agoContributor-Level 10
The escape speed from the Earth's surface is approximately 11.2 km/s. This means that the object must travel at least at this speed in the upwards direction to entirely escape Earth's gravitational pull without falling back.
New answer posted
5 months agoContributor-Level 10
Escape velocity or escape speed is the minimum velocity required for an object to move away from the gravitational pull of a celestial body or planet. This velocity for escape does not require any additional propulsion.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers