Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
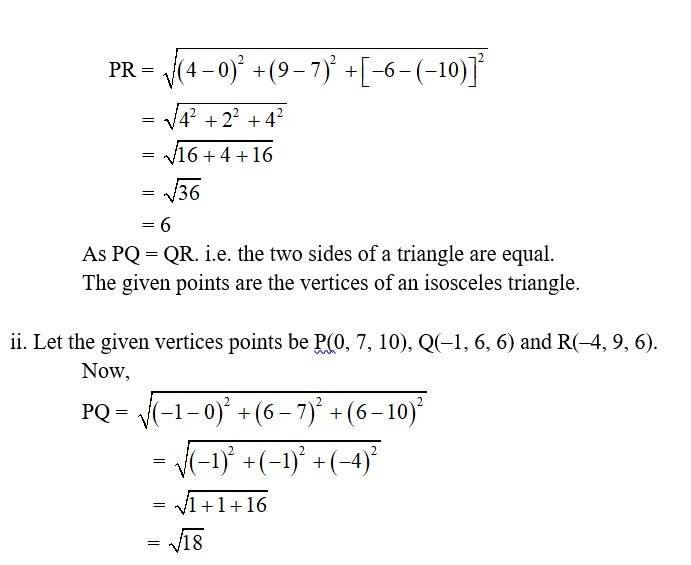
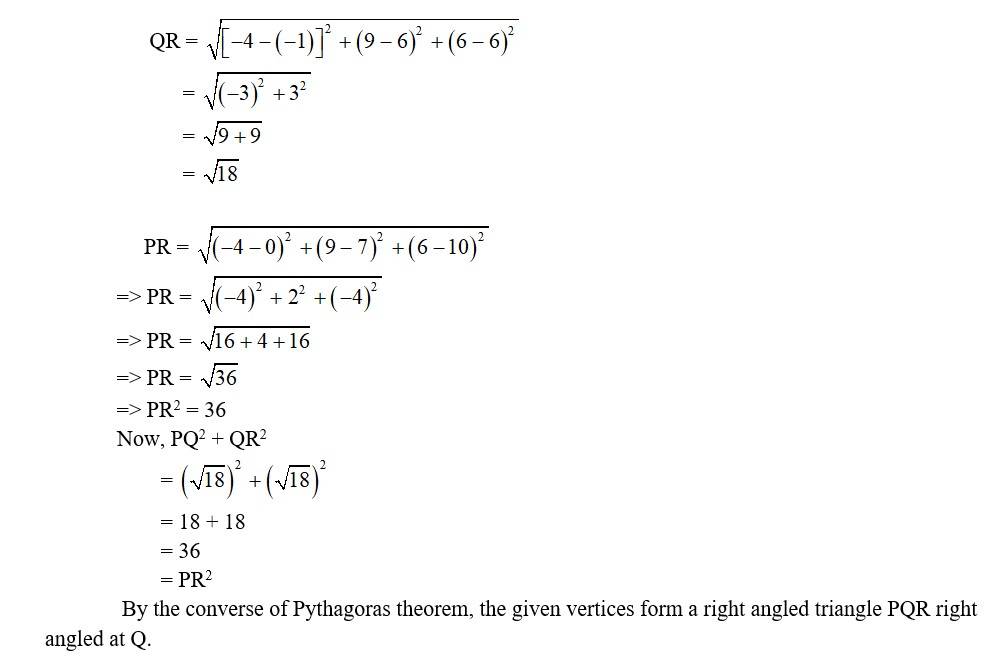
7. i. Let the points be P (0, 7, –10), Q (1, 6, –6) and R (4, 9, –6)
So,
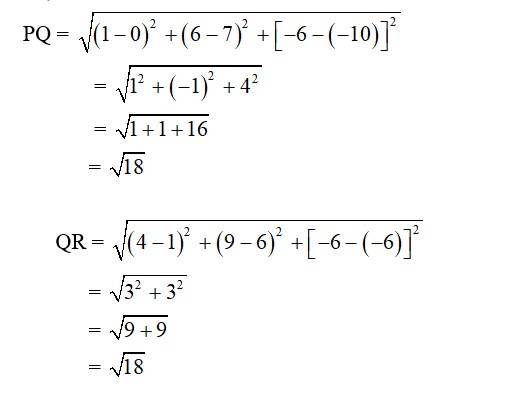

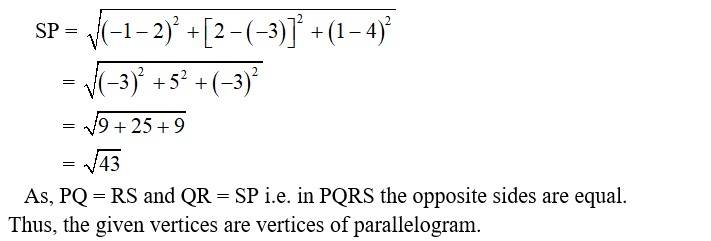
New answer posted
6 months agoContributor-Level 10
6. Here, an=n
Putting n=1,2,3,4,5 we get,
Hence, the first five terms are .
New answer posted
6 months agoContributor-Level 10
3. Here an=2n
Substituting n=1,2,3,4,5 we get,
a1=21=2
a2=22=4
a3=23=8
a4=a4=16
a5=25=32.
Hence the first five terns are 2,4,16,32 and 64.
New answer posted
6 months agoContributor-Level 10
2. Here, a1=
Substituting n=1,2,3,4,5 we get,
.
Hence the first five terns are and .
New answer posted
6 months agoContributor-Level 10
1. Since the ordered pairs are equal, the corresponding elements are equal.
and
y = 1.
New answer posted
6 months agoContributor-Level 10
This is a Long Answer type Questions as classified in NCERT Exemplar
Explanation- mass of helicopter m =2000kg
Mass of the crew and passengers m= 500kg
Acceleration a =15m/s2 and g = 10m/s2
a) force on the floor of the helicopter by the crew and passengers
m (g+a)= 500 (10+15)N= 12500N
b) action of rotor of the helicopter on the surroundings air= (m1+m2) (g+a)
= (2000+500) (10+15)= 2500 (25)= 62500N
c) force on the helicopter due to surroundings air
= reaction of force applied by helicopter
= 62500N
New answer posted
6 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
i. The boiling points of liquid A is approximately 315 K and of liquid B is approximately 345 K (shown below graphically).

ii. Liquid C in a closed vessel will not boil as the pressure keeps on increasing.
New answer posted
6 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
i. CO2 will exist in a gaseous state between the points a and b at temperature T1.
ii. At point b, CO2 will start liquefying when temperature is T1
iii. At point g, CO2 will be completely liquefied when temperature is T2
iv. No, the condensation will not take place when the temperature is T3 because T3 > Tc.
v. Between b and c of the isotherm at T1 , represents the liquid and gaseous CO2 at equilibrium.
New answer posted
6 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
New answer posted
6 months agoContributor-Level 10
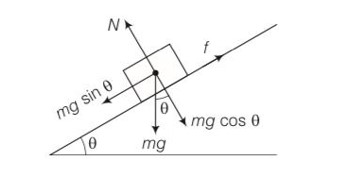
This is a Long Answer type Questions as classified in NCERT Exemplar
Explanation -for the box to just starts sliding down mg
sin
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers