Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
On increasing the pressure slightly the gas 'A' liquefies but gas B does not liquify even on applying high pressure until it is cooled at same conditions of temperature and pressure filled in equal capacity containers it happens because the gas 'A' being at the critical temperature liquifies on slightly increasing the pressure and gas 'B' being at higher temperature than critical temperature does not liquifies even on applying high pressure until it is cooled.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
A gas that follows Boyle's law, Charle's law and Avogadro's law is called an ideal gas.
Real gas behaves ideal under low pressure and high temperature. Under these conditions, the intermolecular interactions are minimum.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The molar volume of both nitrogen and argon at 273.15 K and 1 atm is 22.4 L.
At STP (1 atm pressure and 273.15 K or 00 C), the molar volume i.e the volume of 1 mole gas is 22.4L.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
(a) HCl, HBr and HI have dipole-dipole and london dispersion interaction whereas HF has hydrogen bonding in addition (due to the high electronegativity of the F atom).
(b) The electronegativity decreases from Cl, Br and I so the dipole-dipole interaction will also decrease as HCl > HBr > HI and it is contrary to the boiling point order which is HCl < HBr < HI. This confirms that the London interaction is predominant.
(c) F atom has the highest electronegativity so it has hydrogen bonding interaction in addition to dipole-dipole and london dispersion interaction which leads to the highest boiling poi
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The factors that determine the states of matter are:
1. Temperature
2. Pressure
3. Mass and volume
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Physical state of ice, water and steam are very different but the chemical composition of water in all the three states is H2O.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
(a) the greatest volume is occupied by CH4 and
(b) the smallest volume by NO
According to Avagadro's Law,
Volume of 1 mole of a gas at STP = 22.4 L
Now, we know,
1 mole = Molar mass
∴ Volume of 28g mol-1 of CO at STP = 22.4 L
So, Volume of 1 g of CO at STP = 22.4 L / 28 g mol-1
Similarly,
Volume of 1 g of H2O at STP = 22.4 L / 18 g mol-1
Volume of 1 g of CH4 at STP = 22.4 L / 16 g mol-1 (
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
This is a Long Answer type Questions as classified in NCERT Exemplar
Explanation – as body moving with uniform acceleration a=0
The sum of forces is zero F1+F2+F3=0
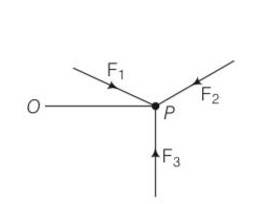
a)let F1, F2, F3 be the three forces passing through the point . let F1and F2 be in the plane A
so F3 =- (F1+F2) so F3 is also in plane A.
b)consider the torque of the forces about P . since all the forces pass through P the torque is zero
torque = OP (F1+F2+F3)
since F1+F2+F3=0 so torque =0
New answer posted
6 months agoContributor-Level 10
This is a Short Answer type Questions as classified in NCERT Exemplar
Explanation -initial speed of coin u= 20m/s
Acceleration of elevator =2m/s2 acceleration due to gravity = 10m/s2
Effective acceleration =g+a=12m/s2
We know that v=u +at
0= 20+ (-12)t
So t= 20/12=5/3 s
Time of ascent = time of descent
Total time coin fall back into hand = (5/3+5/3)=10/3s=3.33s
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
