Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
7 months agoNew answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The reaction is
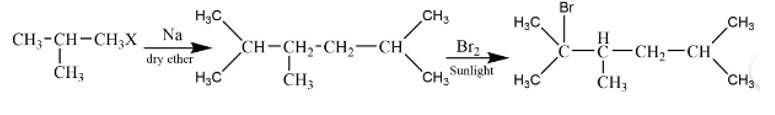
The alkane is
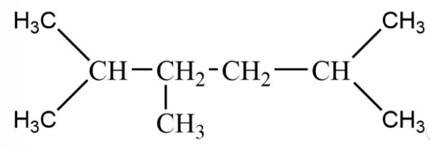
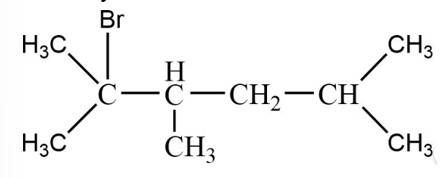
The tertiary bromide is
New answer posted
7 months agoContributor-Level 10
(a) As Fe3+ contains 5 impaired electrons while Fe2+ contains only 4 unpaired electrons. Fe3+ is more paramagnetic.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The 2-methylpropane would lead to two types of radicals.
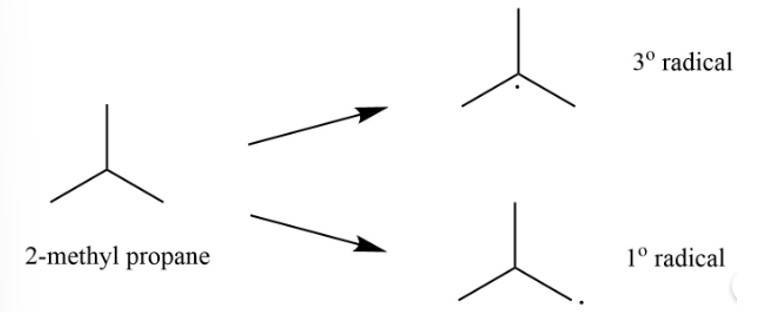
Among the two 3o radical is most stable as it contains 9 ∝ -hydrogen whereas 1o radical contains only 1 ∝ -hydrogen.
New answer posted
7 months agoContributor-Level 10
(b) Both the statements are correct but R is not the reason for A.
New answer posted
7 months agoContributor-Level 10
(a) Fully filled and half-filled orbitals have extra stability (that is, lower energy). Thus, p3, p6, d5, d10, f 7, f14 etc. configurations, which are either half-filled or filled, are more stable. Chromium and copper therefore adopt the d5 and d10 configuration.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
It is a Wurtz reaction where alkane is formed.
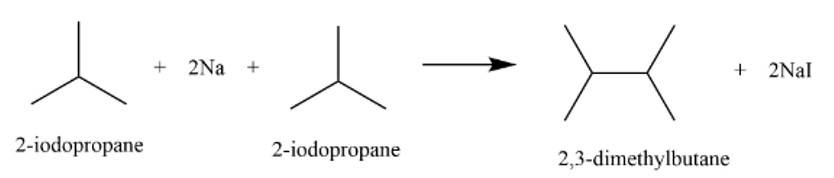
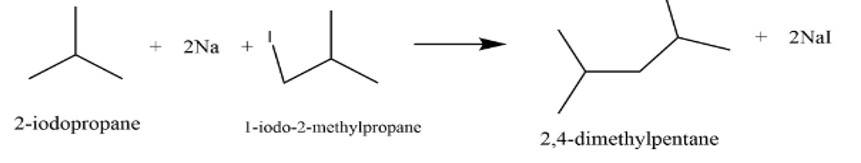
New answer posted
7 months agoContributor-Level 10
(c) The path of an electron in an atom can never be determined or known accurately. That is why there is only a probability of finding the electron at different points in an atom.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The structure of 2-methylbutane is
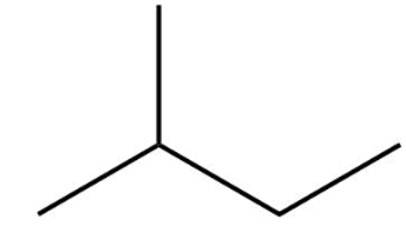
2-methylbutane
Total number | Total amount of monochlorinated Product | % of monochlorinated product | |
1° | 9 | 9 | 41.7 |
2° | 2 | 7.6 | 35.2 |
3° | 1 | 5 | 23.1 |
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
