Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
option (iii)
Standard enthalpy of combustion is defined as the enthalpy change per mole (or per unit amount) of a substance when it undergoes combustion and all the reactants and products being in their standard states at the specified temperature.
New answer posted
7 months agoContributor-Level 10
This is a matching answer type question as classified in NCERT Exemplar
(i) → (d); (ii) → (a); (iii) → (e) : (iv) → (c) : (v) → (b)
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(ii) & (iii)
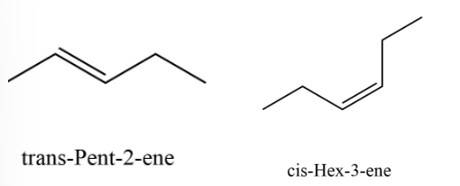
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
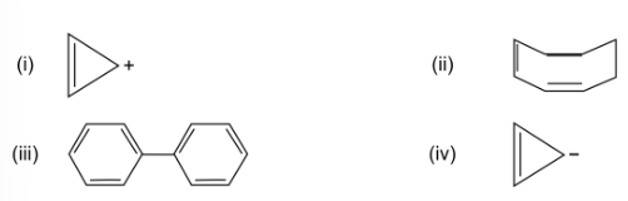
(i) & (iii)
The structures that follow the (4n+2) Huckle rule of aromaticity is considered to be aromatic
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(i) & (iii)
The +I effect of the -OCH3 group stabilizes the carbocation.
The two resonating structure of CH2=CH-CH2⊕↔⊕CH2-CH=CH2 makes it more stable
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(i) & (iii) The nitro group strongly deactivate the ring due to the -R effect and -I effect
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(i) & (iii)
The halogens atom weakly deactivate the ring due to the -I effect and directs the incoming group to the ortho and para position due to the +R effect.
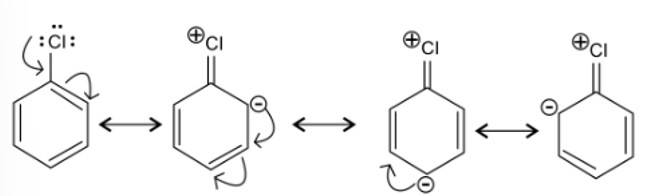
New answer posted
7 months agoContributor-Level 10
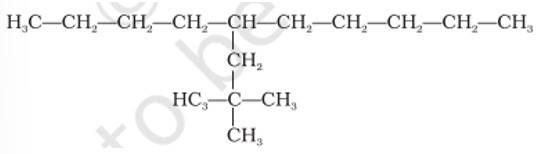
This is a multiple choice answer as classified in NCERT Exemplar
(i) & (iv) The longest carbon chain is of 10 carbon atom
New answer posted
7 months agoContributor-Level 10
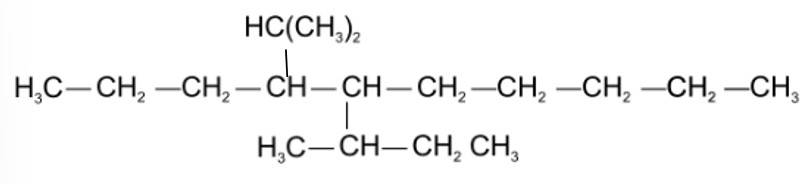
This is a multiple choice answer as classified in NCERT Exemplar
(iii) & (iv) The longest carbon chain is of 10 carbon atom
New answer posted
7 months agoContributor-Level 10
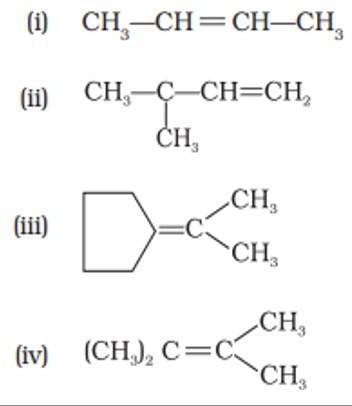
This is a multiple choice answer as classified in NCERT Exemplar
(iii) & (iv) When only the alkyl group is attached to the C atom of the C=C bond then only it would lead to giving only ketone on ozonolysis.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers