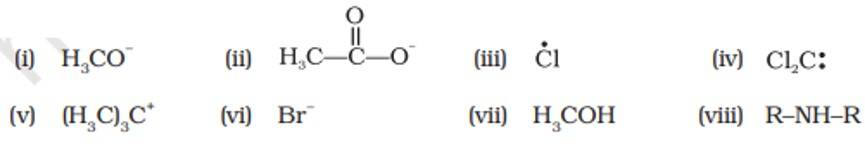Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
(a) Classical mechanics applies to the macro world and hence fails when it is applied to the micro world. In the micro world, quantum mechanics incorporates all the principles like the uncertainty principle, dual behaviour etc.
New answer posted
7 months agoContributor-Level 10
(d) Rainbow is a continuous spectrum.
Assertion is a wrong statement but Reason is a correct one. Rainbow is a continuous spectrum.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Electrophiles | Nucleophiles |
(iii), (iv), (v) | (i), (ii), (vi), (vii), (viii) |
New answer posted
7 months agoContributor-Level 10
(a) For n = 4, No. of sub-shells = (l = 0, l = 1, l = 2, l = 3) = 4.
(b) Total number of orbitals which can be present = n2 = 42 = 16.
Each orbital can have an electron with ms = – 1/2. Total no. of electrons with m, = – 1/2 is 16.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
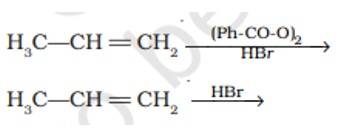
In presence of (Ph-CO-O)2it lead to CH3-CH2-CH2Br as the reaction undergo by free radical mechanism
However in absence of (Ph-CO-O)2 the reaction undergo by carbocation intermediate thus lead to give CH3-CHBr-CH3
New answer posted
7 months agoContributor-Level 10
(a) P (Z=15) : [Ne]103s23p3 No. of unpaired electrons = 3
(b) Si (Z=14) : [Ne]103s23p2 No. of unpaired electrons = 2
(c) Cr (Z=24): [Ar]184s13d5 No. of unpaired electrons = 6
(d) Fe (Z=26): [Ar]184s23d6 No. of unpaired electrons = 4
(e) Kr (Z=36) : [Ar]184s23d104p6 No. of unpaired electrons = Nil.
New answer posted
7 months agoContributor-Level 10
Configuration of the two elements are:
Al (Z = 13): [Ne]103s23p1
Si (Z = 14): [Ne]103s23p2
The unpaired electrons in silicon (Si) will experience more effective nuclear charge because the atomic number of the element Si is more than that of Al.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
New answer posted
7 months agoContributor-Level 10
Greater the penetration of the electron present in a particular orbital towards the nucleus more will be the magnitude of the effective nuclear charge. Based upon this
(i) 2s orbital is closer to the nucleus than 3s orbital and hence it will experience more effective nuclear charge.
(ii) 4d orbital will experience more effective nuclear charge due to its closer proximity to 4f orbital.
(iii) 3p orbital will experience more effective nuclear charge as it is closer to the nucleus
New answer posted
7 months agoContributor-Level 10
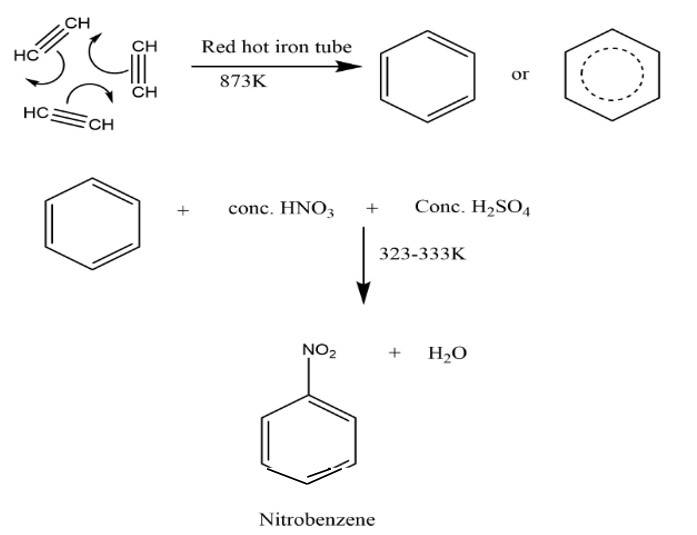
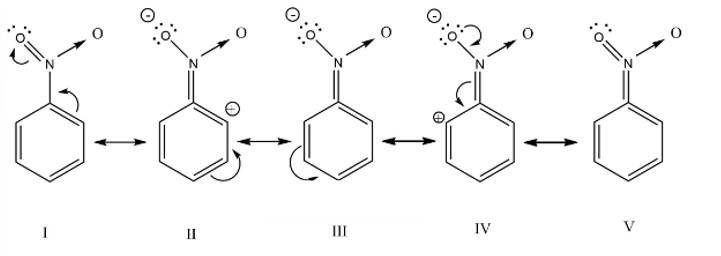
This is a short answer type question as classified in NCERT Exemplar
The nitro group strongly deactivate the benzene towards the electrophilic substitution reaction due to the -R and -I effect
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers