Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Conjugate acid and conjugate base can be shown as-
HCl(aq) + H2O (l) ? H3O+(aq) + Cl-(aq)
acid base conjugate conjugate
acid base
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
It is lithium hydride (LiH) because it has significant covalent character due to the smallest alkali metal, LiH is very stable. It is almost nonreactive towards oxygen and chlorine. It reacts with Al2Cl2 to form lithium aluminium hydride.
8LiH+ Al2Cl6 → 2LiAlH4 → LiCl
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: (a) As we know,
ΔG= ?G? +RTlnQ
?G? = Change in free energy as the reaction proceeds,
ΔG = Standard free energy change,
Q = Reaction quotient,
R = Gas constant,
T = Absolute temperature.
Since, ?G? =−RTlnK
∴ΔG=−RTlnK + RTlnQ = RTlnKQ?
If Q
If Q=K, ΔG=0, reaction is in equilibrium and no net reaction is there.
(b) When we increase the pressure equilibrium will shift in forward direction it means Q
Types of Chemical Reactions :
There are 4 main types of chemical reactions. These are-
- Combination
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
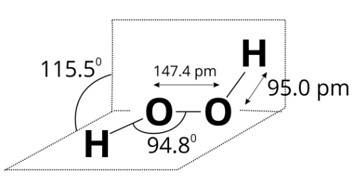
Since, a colourless liquid 'A' contains only hydrogen and oxygen and decomposes slowly on exposure to light but is stabilised by addition of urea, therefore, liquid A may be hydrogen peroxide. A is H2O2.
Structure of H2O2 is given below.
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: The equation can be written as-
Ap+x + Bq- y ? xAp+ (aq) + yBq-
S moles of A and B dissolves to give x S moles of Ap+ and y S moles of Bq-.
Ksp = [Ap+]x [Bq-]y = [x5]x [y5]y
= xx y y 5 x+y
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Mass of H2O2 = 68 g.
Mol. Mass of H2O2=34gmol-1
∴1L of M solution of H2O2 will contain H2O2=34*5 g
∴2L of 5 M solution will contain H2O2=34*5*2=340g
or 200 mL of 5 solution will contain H2O2 = 200 = 34 g
Now 2H2O2 → 2H2O+O2
64 g 32g
Now 68 g of H2O2 on decomposition will give O2=32g
∴34g of H2O2 on decomposition will give O2 = 34 =16 g
New answer posted
7 months agoContributor-Level 10
(i) Industrial preparation: H2O2 is prepared by the auto-oxidation of 2- alkylanthraquinols
2-ethylanthraquinol ↔ H2O2+ oxidised product
2Fe2+ (aq)+2H+ (aq)+H2O2 → Fe3 (aq)+2H2O (l)
PbS (s)+4H2O2 (l) → PbSO4 (s)+4H2O (l)
(ii) Reducing action of hydrogen peroxide
2MnO4-+6H++5H2O2→ 2Mn2++8H2O+5H2
HOCl+H2O2 → H3O++Cl-+O2
Oxidising action of hydrogen peroxide
2Fe2++H2O2→ 2Fe3++2OH-
Mn2++H2O2 → Mn4++2OH-
Acidic properties of H2O2
I2+H2O2+2OH-→ 2I-+2H2O+H2
2MnO4-+3H2O2→ 2MnO2+3O2+2H2O+2OH-
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: According to Le Chatelier's principle, when we raise the temperature, it shifts the equilibrium to left and decreases the equilibrium concentration of ammonia since it is an exothermic reaction. In other words, low temperature and high pressure is favourable for high yield of ammonia. There will be no change in equilibria on addition of argon (Ar).
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: The values of Kc and Qc are itself sufficient to explain the direction of reaction and less than or greater than one another decides the direction in which reaction will proceed as follows-
(i) As Qc < Kc, the reaction proceeds in the forward direction.
(ii) If Qc > Kc, the reaction will proceed in the direction of reactants (reverse reaction).
(iii) If Qc = Kc, no net reaction occurs.
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Hydrogen peroxide is produced by acidifying barium peroxide and eliminating surplus water by evaporation under low pressure. Water is used to extract it, then distillation under lower pressure concentrates it to around 30% (by mass). Careful distillation under low pressure can increase the concentration to 85%. To achieve pure H2O2, the residual water can be frozen out.
Uses of H2O2:
(i) As an antiseptic it is sold in the market as perhydrol.
(ii) It is used to manufacture chemicals like sodium perborate and per - carbonate. It is employed in the industries as a bleachin
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
