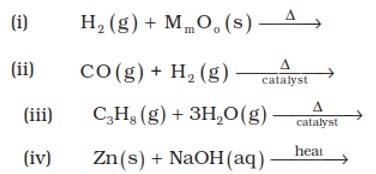- Hydrogen Question and Answers
- kjhgfd
- hhfddh
- 01 Oct 2025
Hydrogen Question and Answers
| 1. Atomic hydrogen combines with almost all elements but molecular hydrogen does not. Explain. |
| Ans: Atomic hydrogen is highly reactive, whereas molecular hydrogen is rather inert. Bond dissociation enthalpy determines the chemical behaviour of dihydrogen (and, for that matter, any molecule) to a great extent. For a single bond between two atoms of any element, the H-H bond dissociation enthalpy is the highest. As a result, molecular hydrogen reacts only with a few elements. |
| 2. How can D2O be prepared from water? Mention the physical properties in which D2O differs from H2O. Give at least three reactions of D2O showing the exchange of hydrogen with deuterium. | |||||||||||||||||||||||||||||||||
| Ans: D2O can be prepared by prolonged electrolysis of water. Due to high molecular mass D2O differs from water. NaOH+D2O → NaOD+HOD HCl+D2O → DCl+ HOD NH4Cl+D2O → NH3DCl+HOD Physical Properties of H2O and D2O
|
| 3. How will you concentrate H2O2? Show differences between structures of H2O2 and H2O by drawing their spatial structures. Also mention three important uses of H2O2. |
| Ans: Hydrogen peroxide is produced by acidifying barium peroxide and eliminating surplus water by evaporation under low pressure. Water is used to extract it, then distillation under lower pressure concentrates it to around 30% (by mass). Careful distillation under low pressure can increase the concentration to 85%. To achieve pure H2O2, the residual water can be frozen out. Uses of H2O2: (i) As an antiseptic it is sold in the market as perhydrol. (ii) It is used to manufacture chemicals like sodium perborate and per - carbonate. It is employed in the industries as a bleaching agent for textiles. (iii) It is used in laboratories as an oxidising agent. Also used in rocket fuels. |
| 4. (i) Give a method for the manufacture of hydrogen peroxide and explain the reactions involved therein. |
| Ans: Industrial preparation: H2O2 is prepared by the auto-oxidation of 2- alkylanthraquinols 2-ethylanthraquinol ↔ H2O2+ oxidised product 2Fe2+(aq)+2H+(aq)+H2O2 → Fe3(aq)+2H2O(l) PbS(s)+4H2O2(l) → PbSO4(s)+4H2O(l) |
| (ii) Illustrate oxidising, reducing and acidic properties of hydrogen peroxide with equations. |
| Ans: Reducing action of hydrogen peroxide 2MnO4-+6H++5H2O2→ 2Mn2++8H2O+5H2 HOCl+H2O2 → H3O++Cl-+O2 Oxidising action of hydrogen peroxide 2Fe2++H2O2→ 2Fe3++2OH- Mn2++H2O2 → Mn4++2OH- Acidic properties of H2O2 I2+H2O2+2OH-→ 2I-+2H2O+H2 2MnO4-+3H2O2→ 2MnO2+3O2+2H2O+2OH- |
Commonly asked questions
(i) Give a method for the manufacture of hydrogen peroxide and explain the reactions involved therein.
(ii) Illustrate oxidising, reducing and acidic properties of hydrogen peroxide with equations.
(i) Industrial preparation: H2O2 is prepared by the auto-oxidation of 2- alkylanthraquinols
2-ethylanthraquinol ↔ H2O2+ oxidised product
2Fe2+ (aq)+2H+ (aq)+H2O2 → Fe3 (aq)+2H2O (l)
PbS (s)+4H2O2 (l) → PbSO4 (s)+4H2O (l)
(ii) Reducing action of hydrogen peroxide
2MnO4-+6H++5H2O2→ 2Mn2++8H2O+5H2
HOCl+H2O2 → H3O++Cl-+O2
Oxidising action of hydrogen peroxide
2Fe2++H2O2→ 2Fe3++2OH-
Mn2++H2O2 → Mn4++2OH-
Acidic properties of H2O2
I2+H2O2+2OH-→ 2I-+2H2O+H2
2MnO4-+3H2O2→ 2MnO2+3O2+2H2O+2OH-
Atomic hydrogen combines with almost all elements but molecular hydrogen does not. Explain.
This is a long answer type question as classified in NCERT Exemplar
Atomic hydrogen is highly reactive, whereas molecular hydrogen is rather inert. Bond dissociation enthalpy determines the chemical behaviour of dihydrogen (and, for that matter, any molecule) to a great extent. For a single bond between two atoms of any element, the H-H bond dissociation enthalpy is the highest. As a result, molecular hydrogen reacts only with a few elements.
How can D2O be prepared from water? Mention the physical properties in which D2O differs from H2O. Give at least three reactions of D2O showing the exchange of hydrogen with deuterium.
This is a long answer type question as classified in NCERT Exemplar
D2O can be prepared by prolonged electrolysis of water. Due to high molecular mass D2O differs from water.
NaOH+D2O → NaOD+HOD
HCl+D2O → DCl+ HOD
NH4Cl+D2O → NH3DCl+HOD
Physical Properties of H2O and D2O
Property | H2O | D2O |
Molecular mass (g mol-1) | 18.015 | 20.0276 |
Melting point/K | 273.0 | 276.8 |
Boiling point/K | 373.0 | 374.4 |
Enthalpy of formation/kJ mol-1 | -285.9 | -294.6 |
Enthalpy of Vaporisation (373 K)/kJ mol-1 | 40.66 | 41.61 |
Enthalpy of fusion/kJ mol-1 | 6.01 | — |
Temp of max. density/K | 276.98 | 284.2 |
Density (298 K)/g cm-3 | 1.0000 | 1.1059 |
Viscosity/centipoise | 0.8903 | 1.107 |
Dielectric constant | 78.39 | 78.06 |
How will you concentrate H2O2? Show differences between structures of H2O2 and H2O by drawing their spatial structures. Also mention three important uses of H2O2.
This is a long answer type question as classified in NCERT Exemplar
Hydrogen peroxide is produced by acidifying barium peroxide and eliminating surplus water by evaporation under low pressure. Water is used to extract it, then distillation under lower pressure concentrates it to around 30% (by mass). Careful distillation under low pressure can increase the concentration to 85%. To achieve pure H2O2, the residual water can be frozen out.
Uses of H2O2:
(i) As an antiseptic it is sold in the market as perhydrol.
(ii) It is used to manufacture chemicals like sodium perborate and per - carbonate. It is employed in the industries as a bleaching agent for textiles.
(iii) It is used in laboratories as an oxidising agent. Also used in rocket fuels.
What mass of hydrogen peroxide will be present in 2 litres of a 5 molar solution? Calculate the mass of oxygen which will be liberated by the decomposition of 200 mL of this solution.
This is a long answer type question as classified in NCERT Exemplar
Mass of H2O2 = 68 g.
Mol. Mass of H2O2=34gmol-1
∴1L of M solution of H2O2 will contain H2O2=34×5 g
∴2L of 5 M solution will contain H2O2=34×5×2=340g
or 200 mL of 5 solution will contain H2O2 = 200 = 34 g
Now 2H2O2 → 2H2O+O2
64 g 32g
Now 68 g of H2O2 on decomposition will give O2=32g
∴34g of H2O2 on decomposition will give O2 = 34 =16 g
A colourless liquid ‘A’ contains H and O elements only. It decomposes slowly on exposure to light. It is stabilised by mixing urea to store in the presence of light.
(i) Suggest possible structure of A.
This is a long answer type question as classified in NCERT Exemplar
Since, a colourless liquid 'A' contains only hydrogen and oxygen and decomposes slowly on exposure to light but is stabilised by addition of urea, therefore, liquid A may be hydrogen peroxide. A is H2O2.
Structure of H2O2 is given below.
An ionic hydride of an alkali metal has significant covalent character and is almost unreactive towards oxygen and chlorine. This is used in the synthesis of other useful hydrides. Write the formula of this hydride. Write its reaction with Al2Cl6.
This is a long answer type question as classified in NCERT Exemplar
It is lithium hydride (LiH) because it has significant covalent character due to the smallest alkali metal, LiH is very stable. It is almost nonreactive towards oxygen and chlorine. It reacts with Al2Cl2 to form lithium aluminium hydride.
8LiH+ Al2Cl6 → 2LiAlH4 → LiCl
Sodium forms a crystalline ionic solid with Dihydrogen. The solid is non-volatile and non-conducting in nature. It reacts violently with water to produce Dihydrogen gas. Write the formula of this compound and its reaction with water. What will happen on electrolysis of the melt of this solid?
This is a long answer type question as classified in NCERT Exemplar
Sodium reacts with Dihydrogen to form sodium hydride which is a crystalline ionic solid.
2Na + H2 → 2Na + H-
It reacts with H2O to produce H2 gas
2NaH+2H2O → 2NaOH+2H2
Although Na+H- does not conduct electricity in the solid state, the electrolysis of its melt produces H2 at the anode and Na at the cathode.
At cathode At anode
Na + H- (l) → 2Na (l) + H2 (g)
Their melts conduct electricity and on electrolysis liberate Dihydrogen gas at anode, which confirms the existence of H-ion.
The Physical Properties of Hydrogen are-
- Hydrogen is a gas with no color and no odor
- Hydrogen has the least density of all gases.
- Hydrogen is viewed as the clean fuel of the future which is developed from water and returned back to water when oxidized.
- Hydrogen is present in almost all molecules in living things.
- It remains connected with carbon and oxygen atoms.
- Hydrogen is the most plentiful element in the whole universe. It is present as a gas in the atmosphere in 1 part per million volume.
- It is spotless, non-toxic, and safe to produce from various sources.
- It is named an energy career because it stores energy that is first created somewhere else.
How can production of hydrogen from water gas be increased by using water gas shift reaction?
This is a short answer type question as classified in NCERT Exemplar
Water-gas shift reaction: The water-gas shift reaction is a chemical reaction in which carbon monoxide reacts with steam in the presence of a catalyst to form carbon dioxide and hydrogen.
CO+H2+H2O → CO2+2H2
What are metallic/interstitial hydrides? How do they differ from molecular hydrides?
This is a short answer type question as classified in NCERT Exemplar
These are formed by many d-block and/block elements. However, the metals of group 7, 8 and 9 do not form hydride. Unlike saline hydrides, they are almost always non- stoichiometric, being deficient in hydrogen. For example, LaH 2.87, YbH 2.55, TiH 1.5-1.8, ZrH 1.3-1.75. Dihydrogen forms molecular compounds with most of the jp-block elements.
Most familiar examples are CH4, NH3, H2O and HF.
Name the classes of hydrides to which H2O, B2H6 and NaH belong.
This is a short answer type question as classified in NCERT Exemplar
NaH belongs to Saline hydrides and B2H6 and H2O belong to molecular hydrides.
If the same mass of liquid water and a piece of ice is taken, then why is the density of ice less than that of liquid water?
This is a short answer type question as classified in NCERT Exemplar
Ice has a highly ordered 3D hydrogen bonded structure. Each oxygen atom is surrounded tetrahedrally by four other oxygen atoms at a distance of 276 pm.
Complete the following equations:
(i) PBS(s)+H2O2(aq) →
(ii) CO(g)+H2(g) →
This is a short answer type question as classified in NCERT Exemplar
(i) PBS (s)+4H2O2 (aq) → PbSO4 (s)+4H2O (l)
(ii) CO (g)+2H2 (g) → CH3OH (l)
Give reasons:
(i) Lakes freeze from top towards bottom.
(ii) Ice floats on water.
(i) During winter, the temperature of lake water keeps on decreasing. Since cold water is heavier, it moves towards the bottom.
(ii) Density of ice is less than that of water that is why it floats on the surface of water.
What do you understand about the term ‘autoprotolysis of water’? What is its significance?
This is a short answer type question as classified in NCERT Exemplar
Self ionization of water is known as autoprotolysis of water.
H2O (l) + H2O (l) → H2O2 (aq) + OH- (aq)
Acid Base Conjugate acid Conjugate acid
Significance of autoprotolysis: Due to autoprotolysis, water has the ability to act as an acid as well as a base. Thus, it behaves as an amphoteric substance.
H2O (l)+NH3 (aq) ↔ OH- (aq)+NH4+ (aq)
H2O (l)+H2 (aq) ↔ H3O+ (aq)+HS- (aq)
Discuss briefly de-mineralisation of water by ion exchange resin.
This is a short answer type question as classified in NCERT Exemplar
Water demineralization using organic or synthetic ion exchange resins (ion exchange resins):
a. Synthetic resins are insoluble polymeric solids that contain a large hydrocarbon network with reactive acidic or basic groups.
These are better than Zeolite since they can remove all kinds of cations and anions from water. Demineralised or deionised water is the product of this process.
b. There are two categories of these:
Resins for cation exchange: Acidic groups such as COOH or SO3H can be found in them. Resin-H+-can be used to represent them.
Mg2+ + 2H-resin → Mg (resin)2+ 2H+
In hard water Cation exchanger
Ca2+ + 2H-resin →Ca (resin)2+2H+
In hard water cation exchanger
Anion exchange resins: They have basic groups such as OH- or NH2.
They may be represented as resin—OH- or resin.
SO42- + 2OH-resin → SO4- (resin)2+ 2OH-
Hard water anion exchanger
Cl- + OH-resin → Cl- resin +OH
Hard water anion exchanger
Molecular hydrides are classified as electron deficient, electron precise and electron rich compounds. Explain each type with two examples.
This is a short answer type question as classified in NCERT Exemplar
(a) Electron deficient hydrides are those that lack sufficient number of electrons to form typical covalent bonds. Group 13 hydrides, for example (BH3, AlH3, etc.).
(b) Electron precise: Electron precise hydrides are those that have the exact number of electrons required to form covalent bonds. Group 14 hydrides, for example (CH4, SiH4, GeH4, SnH4, PbH4 etc.). They are tetrahedral in shape.
(c) Electron rich hydrides: Electron rich hydrides are those that have more electrons than are required to create conventional covalent bonds. Hydrides of groups 15 to 17. (NH3, PH3, H2O, H2S, H2Se, H2Te, HF etc.).
How is heavy water prepared? Compare its physical properties with those of ordinary water.
This is a short answer type question as classified in NCERT Exemplar
Heavy water can be made by electrolyzing water repeatedly or as a by-product in the fertiliser industry. It's a deuterium compound that's used to make other deuterium compounds.
Physical Properties of H2O and D2O
Property | H2O | D2O |
Molecular mass (g mol-1) | 18.015 | 20.0276 |
Melting point/K | 273.0 | 276.8 |
Boiling point/K | 373.0 | 374.4 |
Enthalpy of formation/kJ mol- | -285.9 | -294.6 |
Enthalpy of Vaporisation (373 K)/kJ mol-1 | 40.66 | 41.61 |
Enthalpy of fusion/kJ mol-1 | 6.01 | — |
Temp of max. density/K | 276.98 | 284.2 |
Density (298 K)/g cm-3 | 1.0000 | 1.1059 |
Viscosity/centipoise | 0.8903 | 1.107 |
Dielectric constant/C2/N.m2 | 78.39 | 78.06 |
Write one chemical reaction for the preparation of D2O2.
This is a short answer type question as classified in NCERT Exemplar
Peroxodisulphate, obtained by electrolytic oxidation of acidified sulphate solutions at high current density, on hydrolysis yields hydrogen peroxide.
2HSO4- (aq) → HO3SOOSO3 2HSO4- (aq)+2H+ (aq)+H2O2 (aq)
This method is now used for the laboratory preparation Of D2O2.
K2S2O8 (s)+2D2O (l) → 2KDSO4 (aq)+D2O2 (l)
Calculate the strength of the 5 volume H2O2 solution.
This is a short answer type question as classified in NCERT Exemplar
2H2O2 → 2H2O+O2
5 volume H2O2 means IL of H2O2 will give 5L of O2 at STP.
On the basis of the equation it is clear that 22.7 L of O2 is produced by 68 g of H2O2.
5L of O2 is produced by = = = g of H2O2=14.9g
i.e. 15 g H2O2 dissolved in 1 L solution will give 5 L oxygen or 1.5 g H2O2/100 mL solution will give 500 mL oxygen. Thus 15 g/L or 1.5% solution is known as the 5V solution of H2O2.
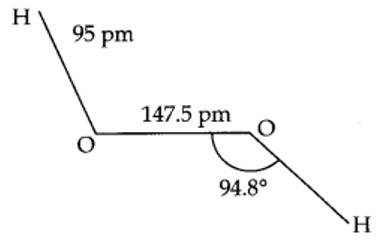
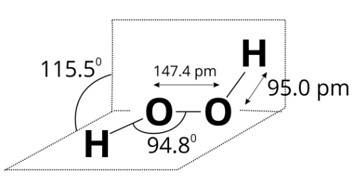
(i) Draw the gas phase and solid phase structure of H2O2.
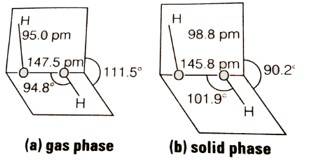
(ii) H2O2 is a better oxidising agent than water. Explain.
This is a short answer type question as classified in NCERT Exemplar
In H2O2 oxygen is in -1 oxidation state which has tendency to become -2 that is why it is a better oxidising agent than water.
Melting point, enthalpy of vaporization and viscosity data of H2O and D2O is given below:
On the basis of this data explain in which of these liquids intermolecular forces are stronger?
This is a short answer type question as classified in NCERT Exemplar
D2O has greater intermolecular force of attraction. This is because D2O exhibits higher values for melting point, enthalpy of vaporization, and viscosity compared to H2O, indicating stronger intermolecular interactions needed to overcome these properties.
Dihydrogen reacts with dioxygen (O2) to form water. Write the name and formula of the product when the isotope of hydrogen which has one proton and one neutron in its nucleus is treated with oxygen. Will the reactivity of both the isotopes be the same towards oxygen? Justify your answer.
This is a short answer type question as classified in NCERT Exemplar
Dihydrogen reacts with dioxygen to form water. The reaction is highly exothermic.
H2 (g) + O2 (g) → H2O (l)
? H = -285.9KJmol-1
The isotope of hydrogen which has one proton and one neutron is deuterium. When it reacts with O2 it forms Deuterium oxide (D2O). Deuterium reacts in a similar way but the reactivity will be lesser than hydrogen because of high bond dissociation enthalpy of D2 as compared to hydrogen.
Explain why HCl is a gas and HF is a liquid.
This is a short answer type question as classified in NCERT Exemplar
Because of hydrogen bonding HF is liquid whereas HCl is gas as there is lack of hydrogen bonding in HCl.
When the first element of the periodic table is treated with dioxygen, it gives a compound whose solid state floats on its liquid state. This compound has an ability to act as an acid as well as a base. What products will be formed when this compound undergoes auto ionization?
This is a short answer type question as classified in NCERT Exemplar
The compound is water which undergoes self ionization.
The autoprotolysis (self-ionization) of water takes place as follows:
H2O (l) + H2O (l) → H3O+ (aq) + OH- (aq)
Acid Base Conjugate acid Conjugate acid
Rohan heard that instructions were given to the laboratory attendant to store a particular chemical i.e., keep it in the dark room, add some urea in it, and keep it away from dust. This chemical acts as an oxidising as well as a reducing agent in both acidic and alkaline media. This chemical is important for use in the pollution control treatment of domestic and industrial effluents.
(i) Write the name of this compound.
(ii) Explain why such precautions are taken for storing this chemical.
This is a short answer type question as classified in NCERT Exemplar
(i) The given compound is H2O2.
(ii) Textiles, paper pulp, leather, oils, fats, and other products use it as a bleaching agent. In both acidic and alkaline media, it works as an oxidising and reducing agent. It is therefore kept in dark wax-lined glass or plastic containers. As a stabiliser, urea might be used. It is kept away from dust since dust can cause the compound to decompose explosively.
Give reasons why hydrogen resembles alkali metals?
This is a short answer type question as classified in NCERT Exemplar
Hydrogen has electronic configuration 1s1. On the one hand, its electronic configuration is similar to that of alkali metals in the first group of the periodic table, which have an outer electronic configuration (ns1). As a result, hydrogen resembles alkali metals that lose one electron to become unipositive ions.
Hydrogen generally forms covalent compounds. Give a reason.
This is a short answer type question as classified in NCERT Exemplar
Because of ionization enthalpy, hydrogen resembles more with halogens, is 520 kJ mol-1, F is 1680 kJ mol-1and that of His 1312 kJ mol-1. Like halogens, it forms a diatomic molecule, combining with elements to form hydrides and a large number of covalent compounds.
Why is the ionisation enthalpy of hydrogen higher than that of sodium?
This is a short answer type question as classified in NCERT Exemplar\
In sodium the last shell electron is in 3s1 after losing that electron, it can acquire the configuration of Ne. Whereas in hydrogen, the electron is in s-orbital.
Basic principle of hydrogen economy is transportation and storage of energy in the form of liquid or gaseous hydrogen. Which property of hydrogen may be useful for this purpose? Support your answer with the chemical equation if required.
This is a short answer type question as classified in NCERT Exemplar
Hydrogen economy (Hydrogen as fuel):
(i) The electricity cannot be stored in automobiles. It is not possible to store and transport nuclear energy. Hydrogen is an alternative source of energy and hence called the 'hydrogen economy". Hydrogen has some advantages as fuel.
(ii) Available in abundance in combined form as water.
(iii) On combustion produces H2O. Hence, pollution free.
(iv) H2O2 fuel cells give more power.
(v) Excellent reducing agent. Therefore, it can be used as a substitute of carbon in reduction for processes in industry.
What is the importance of heavy water?
This is a short answer type question as classified in NCERT Exemplar
Heavy water can be used as a moderator in nuclear reactors and in exchange reactions for the study of reaction mechanisms. It can be prepared by exhaustive electrolysis of water or as a by-product in some fertilizer industries.
Write the Lewis structure of hydrogen peroxide.
This is a short answer type question as classified in NCERT Exemplar
H2O2 structure in gas phase, dihedral angle is 111.50. H2O2 structure in solid phase at 110 k, dihedral angle is 90.20.
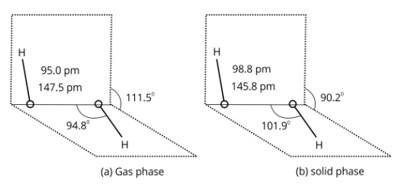
An acidic solution of hydrogen peroxide behaves as an oxidising as well as reducing agent. Illustrate it with the help of a chemical equation.
This is a short answer type question as classified in NCERT Exemplar
(i) Oxidising action in acidic medium:
2Fe2+ (aq)+2H+ (aq)+H2O2 (aq) → 2Fe3+ (aq)+2H2O (l)
PbS (s)+4H2O2 (aq) → PbSO4 (s)+4H2O (l)
(ii) Reducing action in acidic medium:
2MnO4-+6H++5H2O2 → 2Mn2++8H2O+5O2
HOCl+H2O2 → H3O++Cl-+H2O
With the help of suitable examples, explain the property of H2O2 that is responsible for its bleaching action?
This is a short answer type question as classified in NCERT Exemplar
H2O2 decomposes slowly on exposure to light.
2H2O2→ 2H2O+2O2
In daily life it is used as a hair bleach and as a mild disinfectant. As an antiseptic it is sold in the market as perhydrol.
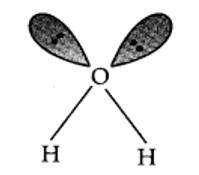
Why are water molecules polar?
This is a short answer type question as classified in NCERT Exemplar
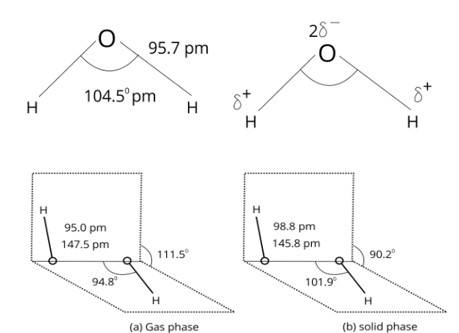
Water molecules are polar because in the gas phase water is a bent molecule with a bond angle of 104.5°, O-H bond length of 95.7 pm. Oxygen is more elsectronegative (EN = 3.5 ) than hydrogen (EN= 2.1 ) hence, O-H bond is polar . In the water molecule, two polar O-H nonds area present which are held together at an angle of 104.5 . Due to the resultant of these two dipoles, water molecule is polar and has an dipole moment of 1.84 Debye.
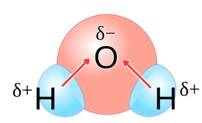
Why does water show a high boiling point as compared to hydrogen sulphide? Give reasons for your answer.
This is a short answer type question as classified in NCERT Exemplar
Water has a significantly higher boiling point than hydrogen sulphide because of the presence of strong hydrogen bonds between water molecules, which are absent in hydrogen sulphide due to the lower electronegativity of sulfur compared to oxygen; this means more energy is required to separate water molecules, resulting in a higher boiling point.
Why can dilute solutions of hydrogen peroxide not be concentrated by heating? How can a concentrated solution of hydrogen peroxide be obtained?
This is a short answer type question as classified in NCERT Exemplar
Hydrogen peroxide cannot be concentrated by heating as it can cause bumps on heating. It can be extracted with water and concentrated to ~30% (by mass) by distillation under reduced pressure. It can be further concentrated to ~85% by careful distillation under low pressure. The remaining water can be frozen out to obtain pure H2O2.
Why is hydrogen peroxide stored in wax lined bottles?
This is a short answer type question as classified in NCERT Exemplar
Hydrogen peroxide is stored in wax lined bottles because decomposes slowly on exposure to light.
Why does hard water not form lather with soap?
This is a short answer type question as classified in NCERT Exemplar
With soap, hard water generates scum/precipitate. Soap containing sodium stearate (C117H35COONa) interacts with hard water to form calcium and magnesium stearate (Ca/Mg stearate). As a result, it is unsuitable for laundering. Because of the deposition of salts in the form of scale, it is also hazardous to boilers.
Phosphoric acid is preferred over sulphuric acid in preparing hydrogen peroxide from peroxides. Why?
This is a short answer type question as classified in NCERT Exemplar
Because H2SO4 can activate the decomposition of H2O2.
How will you account for the 104.5° bond angle in water?
This is a short answer type question as classified in NCERT Exemplar
In water hybridisation of oxygen is sp3. Angle should be109o (approx.) but due to LP - LP repulsion bond angle reduces to 104.5°.
Write redox reaction between fluorine and water.
This is a short answer type question as classified in NCERT Exemplar
2F2 (g)+2H2O → 4H+ (aq)+4F- (aq)+O2 (g)
Write two reactions to explain the amphoteric nature of water.
This is a short answer type question as classified in NCERT Exemplar
H2O (l)+NH3 (aq) → OH- (aq)+NH4+ (aq)
H2O (l)+H2S (aq) → H3O+ (aq)+HS- (aq)
1. Hydrogen resembles halogens in many respects for which several factors are responsible. Of the following factors which one is most important in this respect?
(i) Its tendency to lose an electron to form a cation.
(ii) Its tendency to gain a single electron in its valence shell to attain stable electronic configuration.
(iii) Its low negative electron gain enthalpy value.
(iv) Its small size
This is a multiple choice answer as classified in NCERT Exemplar
option (ii)
Its tendency to gain a single electron in its valence shell to attain stable electronic configuration.
Like halogens (with ns2np5 configuration belonging to the seventeenth group of the periodic table), it is short by one electron to the corresponding noble gas configuration, helium (ls2).
Why does H+ ion always get associated with other atoms or molecules?
(i) Ionisation enthalpy of hydrogen resembles that of alkali metals.
(ii) Its reactivity is similar to halogens.
(iii) It resembles both alkali metals and halogens.
(iv) Loss of an electron from hydrogen atom results in a nucleus of very small size as compared to other atoms or ions. Due to smal size it cannot exist free.
This is a multiple choice answer as classified in NCERT Exemplar
option (iv)
Loss of an electron from a hydrogen atom results in a nucleus of very small size as compared to other atoms or ions. Due to its small size it cannot exist free.
Loss of the electron from hydrogen atom results in nucleus size (H+) of 1.5 × 10-3. This is extremely small as compared to normal atomic and ionic sizes of 50 to 200 pm. As a consequence, H+ does not exist freely and is always associated with other atoms or molecules.
Metal hydrides are ionic, covalent or molecular in nature. Among LiH, NaH, KH, RbH, CsH, the correct order of increasing ionic character is
(i) LiH > NaH > CsH > KH>RbH
(ii) LiH < NaH < KH < RbH < CsH
(iii) RbH > CsH > NaH > KH > LiH
(iv) NaH > CsH > RbH > LiH > KH
This is a multiple choice answer as classified in NCERT Exemplar
Option (ii)
LiH < NaH < KH < RbH < CsH
In ionic hydride as it is formed by the s-Block element, down the group, electropositive character increases.
Which of the following hydrides is electron-precise hydride?
(i) B2H6
(ii) NH3
(iii) H2O
(iv) CH4
This is a multiple choice answer as classified in NCERT Exemplar
Option (iv)
CH4
These hydrides have the required number of electrons to write their conventional Lewis structures.
Radioactive elements emit α, β and γ rays and are characterised by their halflives. The radioactive isotope of hydrogen is
(i) Protium
(ii) Deuterium
(iii) Tritium
(iv) Hydronium
This is a multiple choice answer as classified in NCERT Exemplar
Option (iii)
Tritium
The tritium concentration is about one atom per 1018 atoms of protium. Of these isotopes, only tritium is radioactive and emits low energy beta particles.
Consider the reactions
(A) H2O2 + 2HI → I2 + 2H2O
(B) HOCl + H2O2 → H3O+ + Cl– + O2
Which of the following statements is correct about H2O2 with reference to these reactions? Hydrogen perioxide is ________.
(i) An oxidising agent in both (A) and (B)
(ii) An oxidising agent in (A) and reducing agent in (B)
(iii) A reducing agent in (A) and oxidising agent in (B)
(iv) A reducing agent in both (A) and (B)
This is a multiple choice answer as classified in NCERT Exemplar
Option (ii)
An oxidizing agent in (A) and reducing agent in (B)
H2O2 acts as an oxidising agent in A as it oxidized iodine from -1 to 0, while in B it acts as reducing agent as it reduced chlorine from +1 to -1
The oxide that gives H2O2 on treatment with dilute H2SO4 is —
(i) PbO2
(ii) BaO2 .8H2O + O2
(iii) MnO2
(iv) TiO2
This is a multiple choice answer as classified in NCERT Exemplar
Option (ii)
BaO2 -8H2O
Hydrogen peroxide is produced by acidifying barium peroxide and eliminating excess water by evaporation under low pressure.
Which of the following equations depict the oxidising nature of H2O2?
(i) 2MnO4- + 6H+ + 5H2O2 → 2Mn2+ + 8H2O + 5O2
(ii) 2Fe3+ + 2H+ + H2O2 → 2Fe2+ + 2H2O + O2
(iii) 2I- + 2H+ + H2O2 → I2+ 2H2O
(iv) KIO4 + H2O2→ KIO3 + H2O + O2
This is a multiple choice answer as classified in NCERT Exemplar
Option (iii)
2I- + 2H+ + H2O2 → I2+ 2H2O
As the oxidation state of iodine changes from -1 to 0, this shows peroxide is oxidising in nature.
Which of the following equations depicts the reducing nature of H2O2?
(i) 2[Fe(CN)6]4- + 2H+ + H2O2 → 2[Fe(CN)6]3- + 2H2O
(ii) I2 + H2O2 + 2OH–→ 2I-+ 2H2O + O2
(iii) Mn2+ + H2O2 → Mn4+ + 2OH
(iv) PbS + 4H2O2→ PbSO4 + 4H2O
This is a multiple choice answer as classified in NCERT Exemplar
Option (ii)
I2 + H2O2 + 2OH–→ 2I-+ 2H2O + O2
As iodine is reduced from 0 to -1.
Hydrogen peroxide is _________.
(i) An oxidizing agent
(ii) A reducing agent
(iii) Both an oxidizing and a reducing agent
(iv) Neither oxidizing nor reducing agent.
This is a multiple choice answer as classified in NCERT Exemplar
Option (iii)
Both an oxidizing and a reducing agent
In both acidic and alkaline medium hydrogen peroxide can act as oxidizing and reducing agent.
Which of the following reactions increases production of Dihydrogen from synthesis gas?
(i) CH4(g)+H2O(g) → CO(g)+3H2(g)
(ii) C(g)+H2O(g) → CO(g)+H2(g)
(iii) CO(g)+H2O(g) → CO2(g)+H2(g)
(iv) C2H6+2H2O → 2CO+5H2O
This is a multiple choice answer as classified in NCERT Exemplar
Option (iii)
CO (g)+H2O (g) → CO2 (g)+H2 (g)
The production of dihydrogen can be increased by reacting carbon monoxide of syngas mixtures with steam in the presence of iron chromate as catalyst.
When sodium peroxide is treated with dilute sulphuric acid, we get
(i) Sodium sulphate and water
(ii) Sodium sulphate and oxygen
(iii) Sodium sulphate, hydrogen and oxygen
(iv) Sodium sulphate and hydrogen peroxide
This is a multiple choice answer as classified in NCERT Exemplar
Option (iv)
Sodium sulphate and hydrogen peroxide.
Na2O2+dil H2SO4 → Na2SO4 → Na2SO4+H2O2
Hydrogen peroxide is obtained by the electrolysis of _______.
(i) Water
(ii) Sulphuric acid
(iii) Hydrochloric acid
(iv) Fused sodium peroxide
This is a multiple choice answer as classified in NCERT Exemplar
Option (ii)
Sulphuric acid
2HSO4- (aq) → HO3SOOSO3H (aq) → 2HSO4- (aq)+2H+ (aq) + H2O2 (aq)
Which of the following reactions is an example of use of water gas in the synthesis of other compounds?
(i) CH4(g) + H2O(g) → CO(g) + H2(g)
(ii) CO(g) + H2O(g) → CO2(g) + H2(g)
(iii) CnH2n+2 + nH2O(g) → nCO + (2n+1)H2
(iv) CO(g)+2H2(g) → CH3OH(l)
This is a multiple choice answer as classified in NCERT Exemplar
Option (iv)
CO (g)+2H2 (g) → CH3OH (l)
The reaction shown in equation (iv) shows the synthesis of methanol from water gas.
Which of the following ions will cause hardness in the water sample?
(i) Ca2+
(ii) Na+
(iii) Cl-
(iv) K+
This is a multiple choice answer as classified in NCERT Exemplar
Option (i)
Ca2+
Water becomes 'hard' when it contains calcium and magnesium salts in the form of hydrogen carbonate, chloride, and sulphate.
Which of the following compounds is used for water softening?
(i) Ca3(PO4)2
(ii) Na3PO4
(iii) Na6P6O18
(iv) Na2HPO4
This is a multiple choice answer as classified in NCERT Exemplar
Option (iii)
Na6P6O18
Sodium hexametaphosphate (Na6P6O18), commercially called ‘calgon’, is used to remove hardness from water.
Elements of which of the following group(s) of periodic table do not form hydrides?
(i) Groups 7, 8, 9
(ii) Group 13
(iii) Groups 15, 16, 17
(iv) Group 14
This is a multiple choice answer as classified in NCERT Exemplar
Option (i)
Groups 7,8, 9
Explanation: The metals belonging to group 7, 8 and 9 do not have tendency to form hydrides.
Only one element forms hydride.
(i) Group 6
(ii) Group 7
(iii) Group 8
(iv) Group 9
This is a multiple choice answer as classified in NCERT Exemplar
Option (i)
Group 6
From group 6, only chromium forms CrH.
In the following questions two or more options may be correct.
Which of the following statements are not true for hydrogen?
(i) It exists as a diatomic molecule.
(ii) It has one electron in the outermost shell.
(iii) It can lose an electron to form a cation which can freely exist.
(iv) It forms a large number of ionic compounds by losing an electron.
This is a multiple choice answer as classified in NCERT Exemplar
Option (iii) and (iv)
H+ does not exist freely and is always associated with other atoms or molecules. It has an extremely high ionisation enthalpy and does not have metallic properties under normal conditions, unlike alkali metals.
Dihydrogen can be prepared on a commercial scale by different methods. In its preparation by the action of steam on hydrocarbons, a mixture of CO and H2gas is formed. It is known as
(i) Water gas
(ii) Syngas
(iii) Producer gas
(iv) Industrial gas
This is a multiple choice answer as classified in NCERT Exemplar
Option (i) and (ii)
The combination of CO and H2 is known as water gas. This mixture of CO and H2 is also known as synthesis gas or 'syngas' since it is used to make methanol and other hydrocarbons.
Which of the following statement(s) is/are correct in the case of heavy water?
(i) Heavy water is used as a moderator in nuclear reactor.
(ii) Heavy water is more effective as solvent than ordinary water.
(iii) Heavy water is more associated than ordinary water.
(iv) Heavy water has lower boiling point than ordinary water.
This is a multiple choice answer as classified in NCERT Exemplar
Option (i) and (iii)
Heavy water is utilised as a moderator in nuclear reactors and exchange reactions, and it is more associated with water due to its higher mass.
Which of the following statements about hydrogen are correct?
(i) Hydrogen has three isotopes of which protium is the most common.
(ii) Hydrogen never acts as cation in ionic salts.
(iii) Hydrogen ion, H+, exists freely in solution.
(iv) Dihydrogen does not act as a reducing agent.
This is a multiple choice answer as classified in NCERT Exemplar
Option (i) and (ii)
When an electron is removed from a hydrogen atom, the nucleus decreases to 1.5 × 10-3 pm in size. When compared to usual atomic and ionic sizes of 50 to 200 pm, this is exceedingly small. As a result, H+ does not exist independently and is always bound to other atoms or molecules.
Some of the properties of water are described below. Which of them is/ are not correct?
(i) Water is known to be a universal solvent.
(ii) Hydrogen bonding is present to a large extent in liquid water.
(iii) There is no hydrogen bonding in the frozen state of water.
(iv) Frozen water is heavier than liquid water.
This is a multiple choice answer as classified in NCERT Exemplar
Option (iii) and (iv)
The presence of extensive hydrogen bonding between water molecules gives it remarkable features in the condensed phase (liquid and solid phases). In comparison to H2S and H2Se, this results in a high freezing point, high boiling point, high heat of vaporisation, and high heat of fusion.
Hardness of water may be temporary or permanent. Permanent hardness is due to the presence of
(i) Chlorides of Ca and Mg in water
(ii) Sulphates of Ca and Mg in water
(iii) Hydrogen carbonates of Ca and Mg in water
(iv) Carbonates of alkali metals in water
This is a multiple choice answer as classified in NCERT Exemplar
Option (i) and (ii)
Presence of calcium and magnesium salts in the form of hydrogen carbonate, chloride and sulphate in water makes water 'hard'.
Which of the following statements is correct?
(i) Elements of group 15 form electron deficient hydrides.
(ii) All elements of group 14 form electron precise hydrides.
(iii) Electron precise hydrides have tetrahedral geometries.
(iv) Electron rich hydrides can act as Lewis acids.
This is a multiple choice answer as classified in NCERT Exemplar
Option (ii) and (iii)
Electron precise hydrides have the required number of electrons to write their conventional Lewis structures. All elements of group 14 form such compounds (e.g., CH4) which are tetrahedral in geometry.
Which of the following statements is correct?
(i) Hydrides of group 13 act as Lewis acids.
(ii) Hydrides of group 14 are electron deficient hydrides.
(iii) Hydrides of group 14 act as Lewis acids.
(iv) Hydrides of group 15 act as Lewis bases.
This is a multiple choice answer as classified in NCERT Exemplar
Option (i) and (iv)
All elements in group 13 are Lewis acids because they form electron-deficient compounds. Excess electrons are found as lone pairs in electron-rich hydrides.
These compounds are made up of elements from groups 15-17. (NH3 has one lone pair, H2O has two, and HF has three.) As a result, they act as Lewis bases.
Which of the following statements is correct?
(i) Metallic hydrides are deficient in hydrogen.
(ii) Metallic hydrides conduct heat and electricity.
(iii) Ionic hydrides do not conduct electricity in solid state.
(iv) Ionic hydrides are very good conductors of electricity in solid state.
This is a multiple choice answer as classified in NCERT Exemplar
Option (i), (ii) and (iii)
Ionic hydrides are crystalline, non-volatile and non-conducting in solid state
Correlate the items listed in Column I with those listed in Column II. Find out as many correlations as you can.
|
Column I |
Column II |
|
(i) Synthesis gas |
a. Na2[Na4(PO3)6] |
|
(ii) Dihydrogen |
(b) Oxidising agent |
|
(iii) Heavy water |
(c) Softening of water |
|
(iv) Calgon |
(d) Reducing agent |
|
(v) Hydrogen peroxide |
(e) Stoichiometric compounds of s-block elements |
|
(vi) Salt like hydrides |
(f) Prolonged electrolysis of water |
|
(g) Zn + NaOH |
|
|
(h) Zn + dil. H2SO4 |
|
|
(i) Synthesis of methanol |
|
|
(j) Mixture of CO and H2 |
This is a matching answer type question as classified in NCERT Exemplar
(i) → (j); (ii) → (h); (iii) → (f) : (iv) → (a); (v) → (b); (vi) → (e)
Match Column I with Column II for the given properties/applications mentioned therein.
|
Column I |
Column II |
|
(i) H |
(a) Used in the name of perhydrol. |
|
(ii)H2 |
(b) Can be reduced to dihydrogen by NaH. |
|
(iii)H2O |
(c) Can be used in hydroformylation of olefin. |
|
(iv) H2O2 |
(d) Can be used in cutting and welding. |
This is a matching answer type question as classified in NCERT Exemplar
(i)→ (d); (ii)→ (b); (iii)→ (c); (iv)→ (a)
Match the terms in Column I with the relevant item in Column II.
|
Column I |
Column II |
|
(i) Electrolysis of water produces |
(a) atomic reactor |
|
(ii)Lithium aluminum hydride is used as |
(b) polar molecule |
|
(iii)Hydrogen chloride is a |
(c) recombines on metal surface to generate high temperature |
|
(iv) Heavy water is used in |
(d) reducing agent |
|
(v) Atomic hydrogen |
(e) hydrogen and oxygen |
This is a matching answer type question as classified in NCERT Exemplar
(i)→ (e); (ii)→ (d); (iii)→ (b); (iv)→ (a); (v)→ (c)
Match the items in Column I with the relevant item in Column II.
|
Column I |
Column II |
|
(i) Hydrogen peroxide is used as a |
(a) zeolite |
|
(ii)Used in Calgon method |
(b) perhydrol |
|
(iii)Permanent hardness of hard water is removed by |
(c) sodium hexametaphosphate |
|
(d) propellant |
This is a matching answer type question as classified in NCERT Exemplar
(i) → (b); (ii) → (c); (iii) → (a)
In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A): Permanent hardness of water is removed by treatment with washing soda.
Reason (R): Washing soda reacts with soluble magnesium and calcium sulphate to form insoluble carbonates.
(i) Statements A and R both are correct and R is the correct explanation of A.
(ii) A is correct but R is not correct.
(iii) A and R both are correct but R is not the correct explanation of A.
(iv) A and R both are false.
This is a assertion and reason answer type question as classified in NCERT Exemplar
(i) Statements A and R both are correct and R is the correct explanation of A.
MCl2+Na2CO3→ MCO3+2NaCl
MSO4+Na2CO3→MCO3+Na2SO4
Assertion (A): Some metals like platinum and palladium, can be used as storage media for hydrogen.
Reason (R): Platinum and palladium can absorb large volumes of hydrogen.
(i) Statements A and R both are correct and R is the correct explanation of A.
(ii) A is correct but R is not correct.
(iii) A and R both are correct but R is not the correct explanation of A.
(iv) A and R both are false.
This is a assertion and reason answer type question as classified in NCERT Exemplar
(i) Statements A and R both are correct and R is the correct explanation of A. Since, metals like Pd and Pt adsorbs a large volume of hydrogen, hence, these are used as a storage media for it.
At 300 K, the vapour pressure of a solution containing 1 mole of n-hexane and 3 moles of n-heptane is 550 mm of Hg. At the same temperature, if one more mole of n-heptane is added to this solution, the vapour pressure of the solution increases by 10 mm of Hg. What is the vapour pressure in mmHg of n-heptane in its pure state ?
P? = X? (P? – P? ) + P?
ATQ
550 = 1/4 (P? – P? ) + P?
2200 = P? – P? + 4P?
560 = 1/5 (P? – P? ) + P?
2200 = P? – P? + 5P?
P? + 3P? = 2200
P? + 4P? = 2800
P? = 600
P? = 400 mmHg
If 75% of a first order reaction was completed in 90 minutes, 60% of the same reaction would be completed in approximately (in minutes)
(Take: log 2 = 0.30; log 2.5 = 0.40 )
first order reaction
K = (2.303/t) log (a? / (a? - x)
K = (2.303/90) log (a? / 0.25a? ) = 0.0154
t = 60% = (2.303/K) log (a? / a? ) . (2)
= (2.303 / 0.0154) x (1 - 0.602) = 59.51 mins ≈ 60
The mass of ammonia in grams produced when 2.8 kg of dinitrogen quantitatively reacts with 1 kg of dihydrogen is
N? + 3H? → 2NH?
(2.8 x 10³) / 28 → 0.1 x 10³ mol LR
(1 x 10³) / 2 → 0.5 x 10³ mol
Mass of NH? produced = 0.2 × 10³ × 17 = 3.4 kg = 3400 g
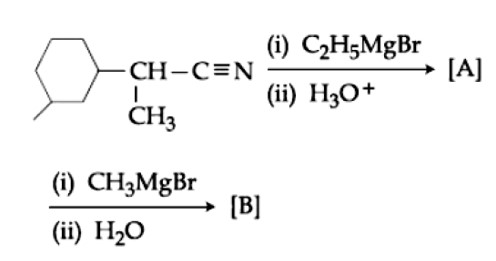
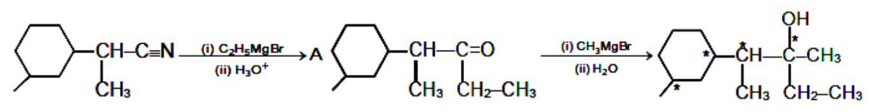
The number of chiral centres present in [B] is
Kindly consider the following Image
At 20.0 mL solution containing 0.2 g impure H2O2 reacts completely with 0.316 g of KMnO4 in acid solution. The purity of H2O2 (in %) is (mol. wt. of H2O2 = 34; mol. wt. of KMnO4 = 158 )
eq of H? O? = eq of KMnO?
(0.316 / 158) x 5 = 0.01
(0.01 x 17 / 0.2) x 100 = 85%
kjhgfd
Students can check the NCERT exercise questions Chemistry Hydrogen, MCQs, and short answer type questions below. Solve these questions after clearing basic concepts.
| 9.5. Describe the bulk preparation of dihydrogen by electrolytic method. What is the role of an electrolyte in this process? |
| Answer: In bulk, dihydrogen can be produced by electrolysis of acidified water using Platinum electrodes. 2H2O (l) → H2 (g) + O2 (g) |
| 9.6. Complete the following reactions. |
| Answer: (i) 3H2(g)+2MoO3 ⟶ Mo2O3+3H2O(l) (ii) CO (g) + H2 (g) ⟶ CH3OH (iii)C3H8 (g) + 3H2O(g) ⟶ 3CO + 7H2(g) (iv) Zn (s) + NaOH (aq) ⟶ Na2ZnO2(s) + H2(g) |
| 9.7. Discuss the consequences of high enthalpy of H-H bond, in terms of chemical reactivity of dihydrogen. |
| Answer: This is due to its small atomic size and small bond length (74 pm) of H-H bond.H−H bond has very high bond enthalpy (435.9 kJ/mol) which results in low reactivity at room temperature. The reactivity is increased at high temperature or in presence of catalyst. Under these conditions, hydrogen reacts with many metals and non-metals to form hydrides. |
| 9.8. What do you understand by (i) electron-deficient, (ii) electron-precise, and (iii) electron-rich compounds of hydrogen? Provide justification with suitable examples. |
| Answer: (i) Electron deficient hydrides: Compounds in which central atom has incomplete octet, are called electron deficient hydrides. For example, BeH2, BH3 are electron deficient hydrides. |
| 9.9. What characteristics do you expect from an electron-deficient hydride with respect to its structure and chemical reactions? |
| Answer: It is expected to be a Lewis acid. They are likely to accept electrons to become stable. They can form coordinate bond with electron rich compound. (Sodium borohydride) |
| 9.10. Do you expect the carbon hydrides of the type (CnH2n+2) to act as ‘Lewis’ acid or base? Justify your answer. |
| Answer: Carbon hydrides of the type CnH2n+2 are electron precise hydrides. Because they have atom with exact number of electrons to form covalent bonds. Thus, they do not behave as Lewis acid or base. Since they have no tendency to accept or lose electrons. |
| 9.11. What do you understand by the term “non-stoichiometric hydrides”? Do you expect this type of hydrides to be formed by alkali metals? Justify your answer. |
| Answer: Those hydrides which do not have fix composition are called non-stoichiometric hydrides, and the composition varies with temperature and pressure. This type of hydrides is formed by d- and f-block elements. They cannot be formed by alkali metals because alkali metal hydrides form ionic hydrides. |
| 9.12. How do you expect the metallic hydrides to be useful for hydrogen storage? Explain. |
| Answer: Metallic hydrides are useful for ultra-purification of dihydrogen and as dihydrogen storage media. In metallic hydrides, hydrogen is adsorbed as H-atoms. Due to the adsorption of H atoms the metal lattice expands and become unstable. Thus, when metallic hydride is heated, it decomposes to form hydrogen and finely divided metal. The hydrogen evolved can be used as fuel. |
| 9.13. How does the atomic hydrogen or oxy-hydrogen torch function for cutting and welding purposes? Explain. |
| Answer: When hydrogen is burnt in oxygen the reaction is highly exothermic, it produces very high temperature nearly 4000°C which is used for cutting and welding purposes. |
| 9.14. Among NH3, H2O and HF, which would you expect to have highest magnitude of hydrogen bonding and why? |
| Answer: HF is expected to have highest magnitude of hydrogen bonding since, fluorine is most electronegative. Therefore, HF is the most polar. |
| 9.15. Saline hydrides are known to react with water violently producing fire. Can CO2, a well known fire extinguisher, be used in this case? Explain. |
| Answer: No. Because if saline hydrides react with water the reaction will be highly exothermic thus the hydrogen evolved in this case can catch fire. CO2 cannot be used as fire extinguisher because CO2 will get absorbed in alkali metal hydroxides. |
| 9.16. Arrange the following: (i) CaH2, BeH2 and TiH2 in order of increasing electrical conductance. (ii) LiH, NaH and CsH in order of increasing ionic character. (iii) H-H, D—D and F—F in order of increasing bond dissociation enthalpy. (iv) NaH, MgH2 and H2O in order of increasing reducing property. |
| Answer: (i) BeH2< TiH2 < CaH2 |
| 9.17. Compare the structures of H2O and H2O2. |
| Answer: In water, O is sp3 hybridized. Due to stronger lone pair-lone pair repulsions than bond pair-bond pair repulsions, the HOH bond angle decreases from 109.5° to 104.5°. Thus, water molecule has a bent structure. |
| 9.18. What do you understand by the term ‘auto-protolysis’ of water? What is its significance? |
| Answer: Auto-protolysis means self-ionisation of water. It may be represented as 2H2O(l) + H2O(l) ⇌ H3O+(aq) + OH-(aq) Acid 1 Base 2 Acid 2 Base 1 |
| 9.19. Consider the reaction of water with F2 and suggest, in terms of oxidation and reduction, which species are oxidised/reduced? |
| Answer: 2F2(ag) + 2H2O(l)→ O2(g) + 4H+(aq) + 4F(aq) |
| 9.20. Complete the following chemical reactions. (i) PbS(s) + H2O2 (aq) → (ii) MnO4– (aq) + H2O2 (aq) → (iii) CaO(s) + H2O(g) → (iv) AlCl3(g) + H2O(l)→ (v) Ca3N2(S) + H2O(l) → Classify the above into (a) hydrolysis, (b) redox and (c) hydration reactions. |
| Answer: (i) PbS(s) +4H2O2(aq) → PbSO4(s) + 4H2O(l) |
| 9.21. Describe the structure of common form of ice. |
| Answer: Ice has crystalline structure which is highly ordered due to hydrogen bonding. It has hexagonal form at atmospheric pressure and cubic form at low temperature. Each O atom has tetrahedral geometry and is surrounded by 4 oxygen atoms each at a distance of 276 pm. |
| 9.22. What causes the temporary and permanent hardness of water? |
| Answer: Temporary hardness of water is due to the presence of bicarbonates of calcium and magnesium in water i.e., Ca(HCO3)2 and Mg(HCO3) in water. Permanent hardness of water is due to the presence of soluble chlorides and sulphates of calcium and magnesium i.e., CaCl2, CaSO4, MgCl2 and MgSO4. |
| 9.23. Discuss the principle and method of softening of hard water by synthetic ion-exchange resins. |
| Answer: Cation exchange resins have large organic molecule with SO3H group which are insoluble in water. Ion exchange resin (RSO3H) is changed to RNa on treatment with NaCl. The resin exchange Na+ ions with Ca2+ and Mg2+ ions present in hard water and make it soft. |
| 9.24. Write chemical reactions to show the amphoteric nature of water. |
| Answer: Water is amphoteric in nature because it acts as an acid as well as a base. |
| 9.25. Write chemical reactions to justify that hydrogen peroxide can function as an oxidising as well as reducing agent. |
| Answer: H2O2 as an oxidising agent: |
| 9.26. What is meant by ‘demineralised’ water and how can it be obtained? |
| Answer: Water free from salts and minerals is called Demineralized water. Ion exchange method is used for this process. The ions present in the water bind to the positively or negatively charged sites on a resin when water is passed through the column packed with resin. |
| 9.27. Is demineralised or distilled water useful for drinking purposes? If not, how can it be made useful? |
| Answer: No, demineralised or distilled water is not fit for drinking purposes. It can be made useful by adding required amount of ions which are useful for our body. |
| 9.28. Describe the usefulness of water in biosphere and biological systems. |
| Answer: (i) Major part of all living system is made of water. (ii) It constitutes about 65 – 70% of body weights of animals and plants. (iii) Some properties of water like high specific heat, thermal conductivity, surface tension, high polarity allow water to play a major role in biosphere. (iv) Because of high heat of vaporisation it is responsible to regulate temperature of living beings. (v) It is an excellent fluid for the transportation of minerals and nutrients in plants. (vi) It is also required for photosynthesis in plants. |
| 9.29. What properties of water make it useful as a solvent? What types of compound can it (i) dissolve, and (ii) hydrolyse? |
| Answer: Water is highly polar in nature. That is why it has high dielectric constant and high dipole moment. Because of these properties, water is a universal solvent. |
| 9.30. Knowing the properties of H2O and D2O, do you think D2O can be used for drinking purposes? |
| Answer: No, D2O is injurious to human beings, plants and animals. |
| 9.31. What is the difference between the terms ‘hydrolysis’ and ‘hydration’? |
| (H+ and OH− ions) of water molecule react with a compound to form products. For example: NaH+H2O → NaOH+H2 Hydration is defined as the addition of one or more water molecules to ions or molecules to form hydrated compounds. For example: CuSO4+5H2O → CuSO4.5H2O |
| 9.32. How can saline hydrides remove traces of water from organic compounds? |
| Answer: Saline hydrides (i.e. CaH2 NaH etc.) react with water and form the corresponding metal hydroxide with the liberation of H2 gas. Thus, these hydrides can be used to remove traces of water from the organic compounds. |
| 9.33. What do you expect the nature of hydrides is, if formed by elements of atomic numbers 15,19, 23 and 44 with dihydrogen? Compare their behaviour towards water. |
| Answer: Atomic number 15 is of phosphorus. The hydride is PH3 and its nature is covalent. Atomic number = 19 is of potassium. The hydride is KH and it is ionic in nature. Atomic number = 23 is of vanadium. The hydride is VH. It is interstitial or metallic. Atomic number =44 is of ruthenium, its hydride is interstitial or metallic. |
| 9.34. Do you expect different products in solution when aluminium (III) chloride and potassium chloride treated separately with (i) normal water (ii) acidified water, and (iii) alkaline water? Write equation wherever necessary. |
| Answer:KCl is a salt of strong acid HCl and strong base KOH. Such salts are neutral in nature and do not undergo hydrolysis. In neutral water, acidic water, and alkaline water, KCl dissociates as KCl → K++Cl− (i) In normal water, it is hydrolyzed. (iii) In alkaline water, the Al(OH)3 obtained from hydrolysis of AlCl3 reacts with hydroxide ions of alkali. The reaction is |
| 9.35. How does H2O2 behave as a bleaching agent? |
| Answer: Bleaching action of H2O2 is due to the oxidation of colouring matter by nascent oxygen. H2O2→ H2O + O |
| 9.36. What do you understand by the terms: (i) Hydrogen economy (ii) hydrogenation (iii) ‘syngas’ (iv) water-gas shift reaction (v) fuel-cell? |
| Answer: (i) Hydrogen economy: The basic principle of hydrogen economy is the storage and transportation of energy in the form of liquid or gaseous dihydrogen. |
hhfddh
| Assertion and Reason: Directions: (a) Assertion and reason both are correct statements and reason is correct explanation for assertion. (b) Assertion and reason both are correct statements but reason is not correct explanation for assertion. (c) Assertion is correct statement but reason is wrong statement. (d) Assertion is wrong statement but reason is correct statement.
9.1.Assertion: H2O2 is stored in wax-lined glass or plastic vessels in dark. Reason:H2O2 decomposes slowly on exposure to light. |
| Answer: (a) H2O2 decomposes slowly on exposure to light. 2 H2O2 (l) → 2H2O(l) + O2 (g) In the presence of metal surfaces or traces ofalkali (present in glass containers), the above reaction is catalysed. It is, therefore, stored inwax-lined glass or plastic vessels in dark. |
| 9.2.Assertion: H+ does not exist freely and is always associated with other atoms or molecules. Reason:H+ form hydrides. |
| Answer:(b) Loss of the electron from hydrogen atomresults in nucleus (H+) of ~1.5×10–3 pm size.This is extremely small as compared to normalatomic and ionic sizes of 50 to 200pm. As a consequence, H+ does not exist freely and isalways associated with other atoms ormolecules. |
| 9.3.Assertion: Lithium hydride used in the synthesis of other useful hydrides. Reason: Lithium hydride is very reactive at moderate temperatures with O2 or Cl2. |
| Answer:(c) Lithium hydride is rather unreactive atmoderate temperatures with O2 or Cl2. It is,therefore, used in the synthesis of other usefulhydrides, e.g., 8LiH + Al2Cl6 → 2LiAlH4 + 6LiCl 2LiH + B2H6 → 2LiBH4 |
| 9.4.Assertion: An ice cube floats on water. Reason: Density of ice is more than that of water. |
| Answer:(c) The crystalline form of water is ice. Atatmospheric pressure ice crystallises in the hexagonal form, but at very low temperatures it condenses to cubic form. Density of ice is less than that of water. Therefore, an ice cube floats on water. In winter season ice formed on the surface of a lake provides thermal insulation which ensures the survival of the aquatic life. This fact is of great ecological significance. |
| 9.5.Assertion: Hard water is desirable for laundry. Reason:Soap containing sodium stearate (C17H35COONa) reacts with hard water to precipitate out Ca/Mg stearate. |
| Answer: (d) Hard water forms scum/precipitate with soap. Soap containing sodium stearate(C17H35COONa) reacts with hard water to precipitate out Ca/Mg stearate.It is, therefore, unsuitable for laundry. It is harmful for boilers as well, because of deposition of salts in the form of scale. This reduces the efficiency of the boiler. |
| MCQs: 9.1. Hydrogen peroxide in the name of perhydrolis used as |
| Answer:(b) antiseptic |
| 9.2. Choose the incorrect statement: (a) Hard water is not suitable for laundry. (b) Temporary hardness can be removed by boiling. (c) Calgon is used for removing permanent hardness of water. (d) Calgon forms precipitates with Ca2+ and Mg2+. |
| Answer: (d) Calgon forms precipitates with Ca2+ and Mg2+. |
| 9.3. Choose the incorrect statement: (a) H+ can exist freely. (b) Hydrogen combines with other elements by losing, gaining, or sharing of electrons. (c) Hydrogen forms electrovalent and covalent bonds with other elements. (d) H+ isextremely small as compared to normal atomic and ionic sizes of 50 to 200 pm. |
| Answer: (a) H+ can exist freely. |
| 9.4. Choose the incorrect statement. (a) H2O2has planar structure. (b) Alkali oxides present in glass catalyse the decomposition of H2O2. (c) Decomposition of H2O2 is a disproportionation reaction. (d) H2O2 molecule simultaneously undergoes oxidation and reduction. |
| Answer: (a) H2O2 has planar structure. |
| 9.5. While converting vegetable oil into vegetable ghee, H2 is used as a (a) oxidising agent (b) reducing agent (c) both (d) none |
| Answer: (b) reducing agent |
| Questions and Answers: 9.1. What property of dihydrogen makes its use in atomic hydrogen torch? |
| Answer:The H–H bond dissociation enthalpy of dihydrogen (435.88 kJ mol–1) is the highest for a single bond between two atoms of any elements. This property is made use of in the atomic hydrogen torch which generates a temperature of ~4000K and is ideal for welding of high melting metals. |
| 9.2. Why is dihydrogen inactive at room temperature? |
| Answer:Dihydrogen is inactive at room temperature because of very highnegative dissociation enthalpy, it combines with almost all the elements under appropriate conditions to form hydrides. |
| 9.3.List the uses of H2O2. |
| Answer: Some of the uses of H2O2 are listed below: (i) In daily life it is used as a hair bleach andas a mild disinfectant. As an antiseptic it is sold in the market as perhydrol. (ii) It is used to manufacture chemicals like sodium perborate and percarbonate,which are used in high quality detergents. (iii) It is used in the synthesis of hydroquinone, tartaric acid and certain food products and pharmaceuticals (cephalosporin) etc. (iv) It is employed in the industries as ableaching agent for textiles, paper pulp,leather, oils, fats, etc. (v) Nowadays it is also used in Environmental(Green) Chemistry. For example, in pollution control treatment of domestic and industrial effluents, oxidation of cyanides, restoration of aerobic conditions to sewage wastes, etc. |
| 9.4. Explain the basic principle of Hydrogen economy. |
| Answer: The basic principle of hydrogen economy is the transportation and storage ofenergy in the form of liquid or gaseous dihydrogen. Advantage of hydrogen economyis that energy is transmitted in the form ofdihydrogen and not as electric power. It is for the first time in the history of India that a pilot project using dihydrogen as fuel was launched in October 2005 for running automobiles.Initially 5% dihydrogen has been mixed in CNG for use in four-wheeler vehicles. The percentage of dihydrogen would be gradually increased to reach the optimum level. Nowadays, it is also used in fuel cells for generation of electric power. It is expected that economically viable and safe sources of dihydrogen will be identified in the years to come, for its usage as a common source ofenergy. |
| 9.5. What are hydrides? Name the different types of hydrides. |
| Answer:Dihydrogen, under certain reaction conditions, combines with almost all elements, except noble gases, to form binary compounds, called hydrides. If ‘E’ is the symbol of an element then hydride can be expressed as EHx (e.g., MgH2) or EmHn (e.g., B2H6). The hydrides are classified into three categories: (i) Ionic or saline or saltlike hydrides (ii) Covalent or molecular hydrides (iii) Metallic or non-stoichiometric hydrides |
| 9.6. What are interstitial hydrides? Give two examples. Write two uses of interstitial hydrides. |
| Answer: Many transition and inner-transition metals absorb hydrogen into the interstices of their lattices to yield metal like hydrides also called the interstitial hydrides. These hydrides are generally non stoichiometric and their composition vary with temperature and pressure. Two uses of interstitial hydrides are: (i) In the storage of H2. |
| 9.7. Water molecule is bent, not linear. Explain? |
| Answer: In water molecule, O is SP3 hybridized. Due to stronger lone pair-lone pair repulsion than bond pair-bond pair repulsions, the H-O-H bond angle decreases from 109.5° to 104.5°. Thus, water is bent molecule. |
| 9.8. What is meant by hard water? How come boiling remove the temporary hardness of water? |
| Answer: Water which does not produce lathers with soap is known as hard water. Hardness is due to the presence of bicarbonates, sulphates and chlorides of Ca2+ and Mg2+. On boiling, the bicarbonates of calcium and magnesium decompose to insoluble carbonate which can be removed by filtration. |
| 9.9. Give two advantages of using hydrogen over gasoline as a fuel. |
| Answer: 1. High heat of combustion.
|
| 9.10. How is dihydrogen prepared from water by using a reducing agent? |
| Answer: Dihydrogen is prepared from water by the action of alkali metals like Na and K which is a strong reducing agent. 2Na + 2H2O → 2NaOH + H2 2K + 2H2O → 2KOH + H2 |
Commonly asked questions
In bromination of Propyne, with Bromine 1, 1, 2, 2-tetrabromopropane is obtained in 27% yield. The amount of 1, 1, 2, 2- tetrabromopropane obtained from 1g of Bromine in this reaction is_______________× 10-1g. (Nearest integer) (Molar mass: Bromine = 80g / mol)
Reaction is
Mass of product, 1, 2, 2- tetrobromopropane obtained
=
= 3.30375
= 3.0375 × 10-1
01 Oct 2025
01 Oct 2025
Chemistry NCERT Exemplar Solutions Class 11th Chapter Nine Exam