Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Mathematical Reasoning Solutions Type Questions as classified in NCERT Exemplar
The three examples of sentences which are not statements are:
(1) He is an engineer.
Here, in this sentence, it is not evident as to whom 'he' is referred to. Hence, it is not a statement.
(2) Painting is easy for some people, painting can be easy and for some others, it can be difficult. Hence, it is not a statement.
(3) Where is my hat?
This sentence is a question which is not considered as a statement in mathematical language. Hence, it is not a statement.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (i) and (ii)
The species having the same number of electrons are known as isoelectronic species.
The number of electrons in CO species is 14; 8 electrons from oxygen atom and 6 electrons from carbon atom.
The number of electrons in NO+ is 14; 8 electrons from oxygen atom, 7 electrons from nitrogen atom and 1 electron will be subtracted due to the positive charge on the species.
The number of electrons in N2 is 14 ; 7 from each nitrogen atom.
The number of electrons in SnCl2 is 84 ;50 from the tin atom and 17 from each chlorine atom.
The number of electrons in NO2
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (i) and (iv)
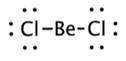
The structures of NO2 and NCO+ has a lone pair of electron on nitrogen atom thus it will form a bent shape rather than a linear shape.
New answer posted
7 months agoContributor-Level 10
This is a Mathematical Reasoning Solutions Type Questions as classified in NCERT Exemplar
(i) This sentence is false because there are less than 35 days in a month. Hence it is a statement.
(ii) This sentence differs to individual, as some may find it difficult and some may find it easy. This means that it is not always true. Hence it is not a statement.
(iii) This sentence is true as the sum of 5 and 7 is 12 which is greater than 10. Hence it is a statement.
(iv) This sentence can be correct and incorrect sometimes as the square of 2 is even but the square of 3 is an odd. Hence it is not a statement.
(v) This sentence is no
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (i) and option (ii)
Bond order depends on the electronic configuration of the molecular species, and the electronic configuration depends on the number of electrons a particular atom is contributing in its molecular structure.
The CN- species has a total of 14 electrons; 6 electrons from carbon atom, 7 electrons from nitrogen atom and 1 electron for the negative charge.
The NO+ species has a total of 14 electrons; 8 from oxygen atoms, 7 from nitrogen atom and 1 electron will be subtracted due to the positive charge on the species.
The O2 - species has a total
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (ii)
Aluminium has [Ne]3s2 3p1 electronic configuration. Phosphorus has [Ne]3s2 3p3. The electronic configuration of silicon is [Ne]3s2 3p2 and the electronic configuration of arsenic is [Ar]3d104s24p3.
Ionisation energy increases as the atomic number in a period increases and decreases on moving down the group. It is also known that the ionisation enthalpy of group 15 elements is more than group 16 elements as group 15 has half-filled p-subshells which gives extra stability.Thus, the electronic configuration having the highest ionization entha
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (i)
The most electronegative element in the periodic table is fluorine having an atomic number 9 . The electronic configuration of fluorine is 1s2 2s2 2p5. Hence, the electronic configuration of the outermost shell of fluorine is 2s2 2p5 .
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (ii)
The electronic configurations and the bond orders of the given species are given below:
Bond order of O2 - : σ1s2 σ∗1s2 σ2s2 σ∗2s2 σ2p2z (π2p2x = π2p2y) (π∗2p1x= π∗2p1y)
Bond order = ( Nb- Na)
BO= (10-7)= = 1.5
Bond order of O2 + : σ1s2 σ∗1s2 σ2s2 σ∗2s2 σ2p2z (π2p2x = π2p2y) (π∗2p1x= π∗2p0y)
Bond order = (10-5)= =2.5
Bond order of O2 : σ1s2 σ∗1s2 σ2s2 σ∗2s2 σ2p2z (π2p2x = π2p2y) (π∗2p1x= π∗2p1y)
BO= (10-6) = =2
So, the correct order of bond order is (ii) O2 – < O2 < O2 +
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iv)
(a) A molecule which has a zero bond order never exists and a molecule having a non-zero bond order either exists or is expected to exist.
(b) If the value of bond order is high then the bond strength will also be higher. Electrons present in the bonding molecular orbital are known as the bonding molecular orbital ( Nb ) and the electrons present in the anti-bonding molecular orbital are known as anti-bonding electrons ( Na ). The half of the difference of bonding and anti-bonding electrons is known as the bond order.
The bond order of Be2 : Be2=s 1s2, s
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iv)
In the molecules of boron ( B2 ), carbon ( C2 ) and nitrogen ( N2 ) the energy of s2pz molecular orbital is greater than the energy of P2px and P2py molecular orbital.
So, the correct order of energies of molecular orbital of N2 is
(π2py ) > (σ2pz ) < (* 2px ) ≈ (π*2py )
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
