Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Mendeleev's arranged the elements as the periodicity of their atomic weights.
The drawbacks of Mendeleev's periodic table are as follows:-
(a) The position of hydrogen in the periodic table is not specified
(b) Isotopes are not included in the periodic table
(c) Elements with higher atomic mass are placed before the elements with lower atomic mass. For e.g- Co & Ni
(d) Gaps are left in his table considering the fact that more elements are yet to be discovered
(e) Inappropriate position of group VII.
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
The group 1 elements are called alkali metals which possess outermost electronic configuration as ns1 .All the elements that have same outermost electron configuration possess similar properties and are placed in the same group.
Atomic number | Symbol | Electronic Configuration |
|
3 | Li | 1s2 2s1 | [He]2s1 |
11 | Na | 1s2 2s2 2p6 3s2 | [Ne]3S1 |
19 | K | 1s2 2s2 2p6 3s2 4s1 | [Ar]4S1 |
37 | Rb | 1s2 2s2 2p6 3s2 3d10 4s2 4p6 5s1 | [Kr]5S1 |
55 87 | Cs Fr | 1s2 2s2 2p6 3s2 3d10 4s2 4p6 5d10 5s2 5p6 6s1 | [Xe]6s1 [Rn]7s1 |
New question posted
7 months agoNew answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
When the gas is compressed adiabatically, the total work done on the gas increases its internal energy which in turn increases the KE of the gas molecule and hence, the collisions between molecules also increases.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
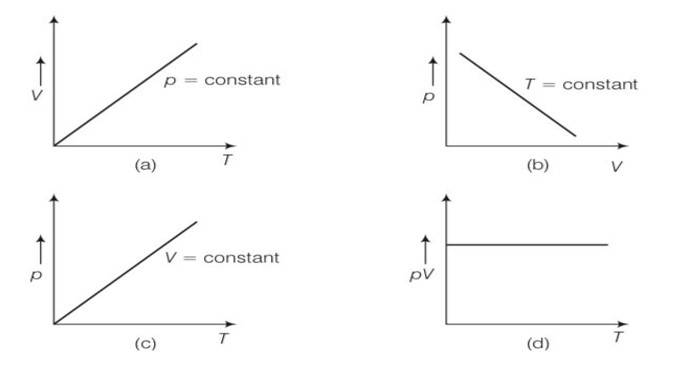
(a), (c) pV=nRT
(a) when pressure p =constant
volume is directly proportional to temperature
(b) when T= constant
from PV =constant
so the graph is rectangular hyperbola
(c) when V =constant
from pressure is directly proportional to temperature.
So the graph is straight the passes through the origin.
PV
PV/T=constant
So the graph hence through origin. So d is correct.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(a), (d) The total energy associated with the molecule is
E=
The number of independent terms in the above expressions is 5. So we can predict velocities of molecule by maxwell's distribution, hence the above expression obeys maxwell 's distribution .
So 2 rotational and 3 translational energies are associated with each molecule
So rotational energy at a given temperature is 2/3 of the translational KE of each molecule.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(c) According to kinetic theory, we know walls only exert perpendicular forces on molecule. They do not exert parallel force . so there is only change in translational motion.
So pV=2/3E
Where E is representing only translational part of energy.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
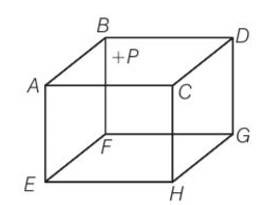
(b), (d) due to the presence of external positive charge on face ABCD. The usual expression for pressure on the basis of kinetic energy will not be valid as ions would also experience electrostatic force. So presence of positive charge the isotropy is also lost.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(d) pV=nRT
So n= PV/RT
As number moles are fixed
P2=p1V1 (T2/V2T1)
=
= P
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(b) For a function f (v), the number of molecules n=f (v) which are having speeds between v and v+dv
So f1 (v) and f2 (v) will obey's maxwell distribution law
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers