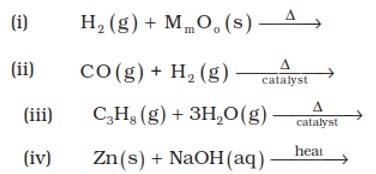Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
9.14. HF is expected to have the highest magnitude of hydrogen bonding since, fluorine is most electronegative. Therefore, HF is the most polar.
New answer posted
8 months agoContributor-Level 10
9.13. When hydrogen is burnt in oxygen the reaction is highly exothermic, it produces very high temperature nearly 4000°C which is used for cutting and welding purposes.
New answer posted
8 months agoContributor-Level 10
9.12. Metallic hydrides are useful for ultra-purification of dihydrogen and as dihydrogen storage media. In metallic hydrides, hydrogen is adsorbed as H-atoms. Due to the adsorption of H atoms the metal lattice expands and become unstable. Thus, when metallic hydride is heated, it decomposes to form hydrogen and finely divided metal. The hydrogen evolved can be used as fuel.
New answer posted
8 months agoContributor-Level 10
9.11. Those hydrides which do not have fix composition are called non-stoichiometric hydrides, and the composition varies with temperature and pressure. This type of hydrides is formed by d- and f-block elements. They cannot be formed by alkali metals because alkali metal hydrides form ionic hydrides.
New answer posted
8 months agoContributor-Level 10
9.10. Carbon hydrides of the type CnH2n+2 are electron precise hydrides. Because they have atom with exact number of electrons to form covalent bonds. Thus, they do not behave as Lewis acid or base. Since they have no tendency to accept or lose electrons.
New answer posted
8 months agoContributor-Level 10
9.9. It is expected to be a Lewis acid. They are likely to accept electrons to become stable. They can form coordinate bond with electron rich compound.
2NaH(s) + B2H6 (g) → 2Na+[BH4]- (s)
&n
New answer posted
8 months agoContributor-Level 10
9.8. (i) Electron deficient hydrides: Compounds in which central atom has incomplete octet, are called electron deficient hydrides. For example, BeH2, BH3 are electron deficient hydrides.
(ii) Electron precise hydrides: Those compounds in which exact number of electrons are present in central atom or the central atom contains complete octet are called precise hydrides e.g., CH4, SiH4, GeH4 etc. are precise hydrides.
(iii) Electron rich hydrides: Those compounds in which central atom has one or more lone pair of excess electrons are called electron rich hydrides, e.g., NH3, H2O.
New answer posted
8 months agoContributor-Level 10
9.7. This is due to its small atomic size and small bond length (74 pm) of H-H bond.H? H bond has very high bond enthalpy (435.9 kJ/mol) which results in low reactivity at room temperature. The reactivity is increased at high temperature or in presence of catalyst. Under these conditions, hydrogen reacts with many metals and non-metals to form hydrides.
New answer posted
8 months agoContributor-Level 10
9.6. (i) 3H2?(g)+2MoO3? ? Mo2?O3?+3H2?O(l)
(ii) CO (g) + H2 (g) ? CH3OH
(iii) C3H8 (g) + 3H2O(g) ? 3CO + 7H2(g)
(iv) Zn (s) + NaOH (aq) ? Na2ZnO2(s) + H2(g)
New answer posted
8 months agoContributor-Level 10
9.5. In bulk, dihydrogen can be produced by electrolysis of acidified water using Platinum electrodes.
2H2O (l) → H2 (g) + O2 (g)
Electrolyte is added to increase the dissociation of water.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers