Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Kf = 1.86
Using, density of water = 1g / mL
i = ?
i = 1.344
Now, using
n for ClCH2COOH = 2
a =
Using
So; x = 36
New question posted
3 months agoNew answer posted
3 months agoContributor-Level 9
According to Gauss's law for magnetism, the net magnetic flux through any closed surface is zero.
It states that all magnetic field lines must enter and exit from a surface. In this way, it differs from electric charges, which can exist independently as positive and negative.
New answer posted
3 months agoContributor-Level 10
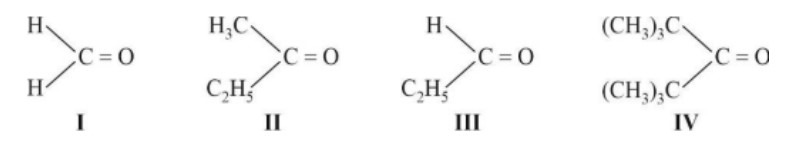
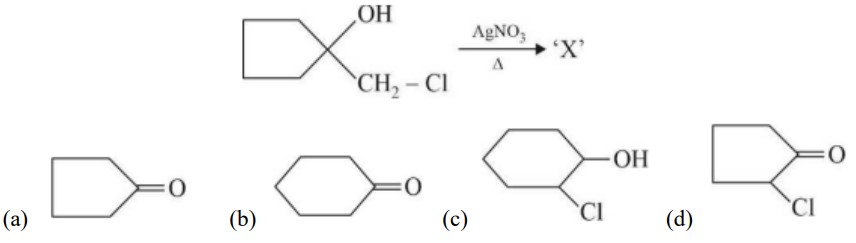
If the carbonyl compound is sterically crowded, then it will be reluctant to undergo addition reaction. Moreover, attachment of bulkier alkyl group with the carbonyl carbon decreases the partial positive charge resulting into the minimization of attack by R? from RMgBr. So, the order is
New answer posted
3 months agoContributor-Level 9
Magnetism is the force exerted by a magnet. It comes when the magnet attracts or repulses an object. For example, a magnet sticks to certain objects because the magnetic force or magnetism pulls it.
New answer posted
3 months agoContributor-Level 10
Stability constant are :
K1 = 104
K2 = 1.58 * 103
K3 = 5 * 102
K4 = 102
Overall stability constant K will be
K = K1 * K2 * K3 * K4
= 7.9 * 1011
Now, overall equilibrium constant for dissociation of [Cu (NH3)4]2+ is
= 1.26 * 10-12
So; x = 1 (Rounded off to the nearest integer)
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers