Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
In case 1, E? = 2eV; φ? ? = E? - KE? = 1eV
In case 2, E? = 12400/1550 = 8eV; max.KE = 8eV - 1eV = 7eV
New answer posted
3 months agoContributor-Level 9
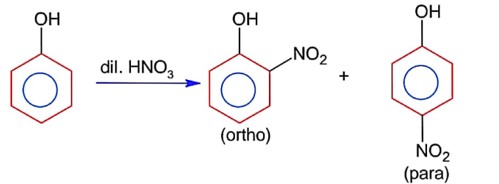
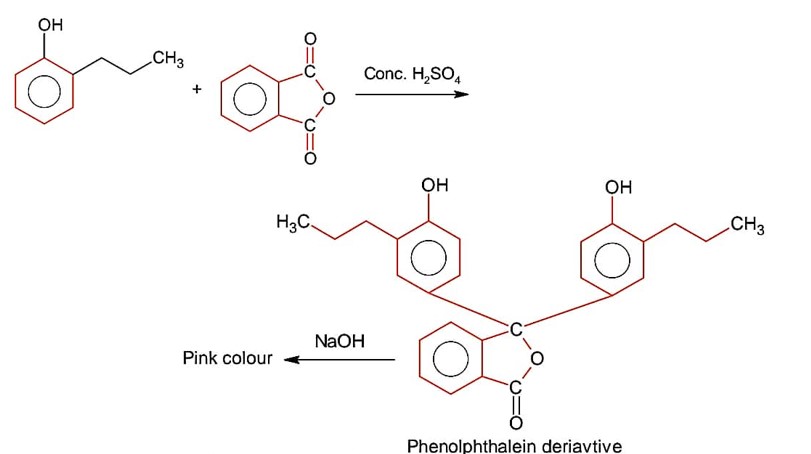
Ortho product has intramolecular H- bonding & para product has intermolecular H- bonding. Thus it can be separated by steam distillation due to difference in B.
New answer posted
3 months agoContributor-Level 10
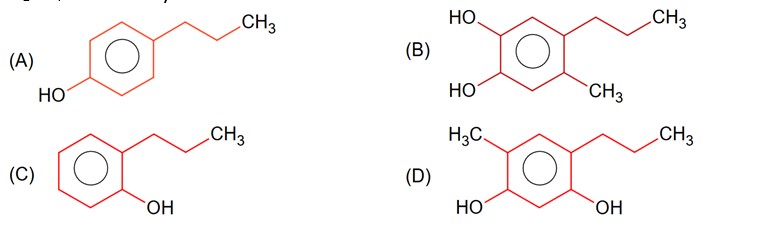
First reaction is EAS which will be given by that phenol derivative whose p-position is free for EAS reaction.
New answer posted
3 months agoContributor-Level 9
the strongest oxidizing agent have the highest reduction potential. So Mn3+ is the strongest oxidizing agent.
New answer posted
3 months agoContributor-Level 9
Cerium exists in two oxidation states (+3) and (+4)
It exist as Ce+4 and acts like a strong oxidizing agent by gaining electrons
New answer posted
3 months agoContributor-Level 10
In y axis u? = v? a? = -E? q / m
s? = 0, u? t + (1/2)a? t² = 0 ⇒ t = 2u? /a? = 2v? m/E? q
x coordinate at that time = v? * t = (2v? m/0) * v? = (2v? ²m)/E? q
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers