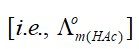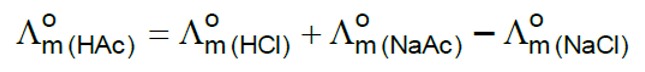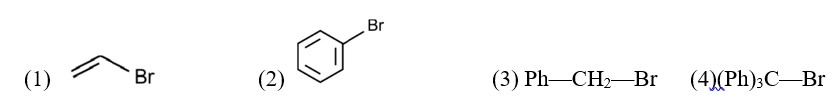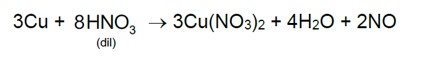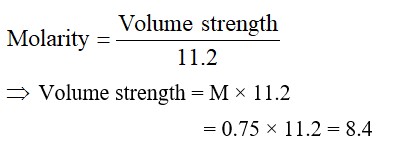Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
2 months agoContributor-Level 10
AICI3 has greater number of ions, therefore it shows the highest osmotic pressure.
New answer posted
2 months agoNew answer posted
2 months agoContributor-Level 10
Charge passed = It
= 0.015 * 15 * 60 C
Moles of electrons passed
Moles of Zn deposited =
= 0.00007
Mass of Zn deposited = 0.00007 * 65.4 g = 4.58mg
New answer posted
2 months agoContributor-Level 10
After the 2023-24 revised CBSE syllabus, many chapters have been removed, and in some chapters syllabus is reduced. As per the latest CBSE exam pattern 2025-26, the Chapter 1 Solutions will be asked for 5-7 marks in the Class 12 Chemistry CBSE board exams.
For other competitive exams, the Solutions chapter has a medium weightage in the NEET exam. In the NEET exam, 1-2 questions are frequently asked based on the concept of moderate-level numerical.
Physical Chemistry holds over 30% weightage in the chemistry section in the JEE Mains exam. The solutions chapter is considered a part of physical chemistry. You can expect 1 question fo
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 680k Reviews
- 1800k Answers