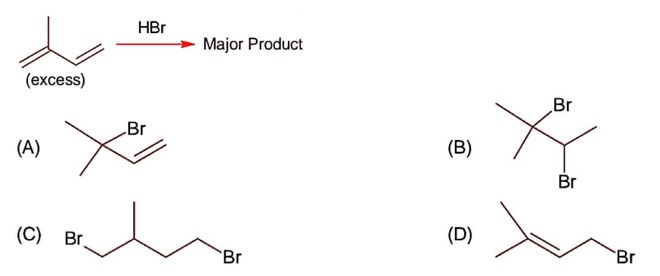
Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
During adsorption of a gas on solid surface, process is exothermic i.e. and entropy decreased, because gas particle resides on solid surface. Hence
New answer posted
5 months agoContributor-Level 10
{for ethylene glycol I = 1, due to non-ionisable}
(the nearest integer)
Hence, freezing point of solution = 273 K – 4K = 269 K.
New answer posted
5 months agoContributor-Level 10
Total number of excellent students = 120
Number of female excellent students = 70
Required fraction
New answer posted
5 months agoContributor-Level 10
Sphalerite is the sulphide ore of zinc (ZnS) and copper glance Cu2S is the sulphide ores of copper. Two sulphide ores can be separated by adjusting proportion of oil to water or by using depressant in froth flotation method.
New answer posted
5 months agoContributor-Level 10
Number of female good students = 8
Number of males average students = 48
Required ratio = 8 : 48 = 1 : 6
New answer posted
5 months agoContributor-Level 10
Photo-chemical smog is the mixture of various component like, O3, NO, acrolein formaldehyde and peroxyacetyl nitrate and other oxidizing agents, which causes the cracking of rubber.
New answer posted
5 months agoContributor-Level 10
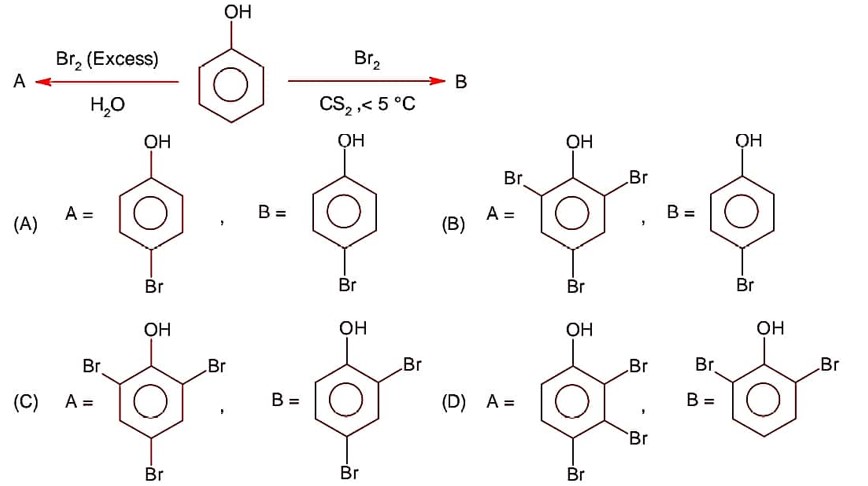
CS2 is non-polar, non-ionisable solvent and produce only para substituted compound due to the production of less Br+ ion, while H2O itself a polar solvent and produce sufficient concentration of Br+ to attack on ortho and para position.
New answer posted
5 months agoContributor-Level 10
From the above table, it is clear that number of female good students = 8
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers