Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
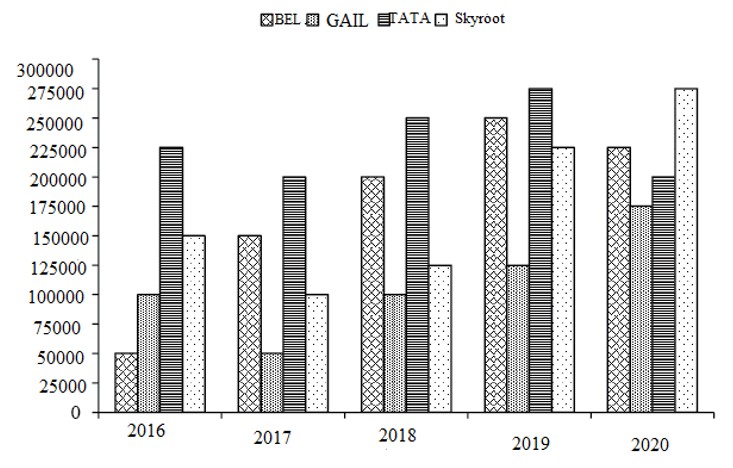
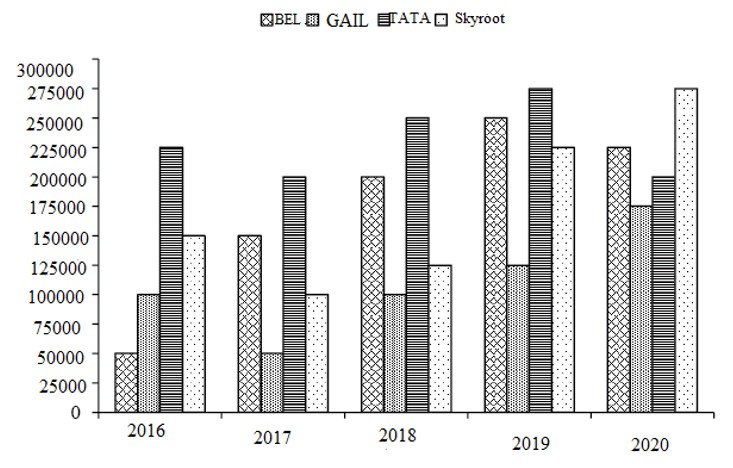
Skyroot's income was the maximum in year 2020, as the rate charged was the maximum and the number of units was also the maximum
New answer posted
5 months agoContributor-Level 10
Weight = 1.53 gm
V = 448 ml (STP)
Mole =
Molecular weight of compound A = 50 * 1.53 = 76.5 gram
Molecular weight 76.5 = CnH2n-1 Cl
or 12 n + 2n – 1 = 41
Molecular formula = C3H5Cl
Number of C- atoms = 3
New answer posted
5 months agoContributor-Level 10
In Frenkel defect, cations are inserted in neighbouring site, therefore statement-I is correct, because vacancy developed by missing cations and interstitial sites are equal. Statement II is false because F-centre developed by metal excess defect due to anion vacancies.
New answer posted
5 months agoContributor-Level 10
Violet colour
Blood red colour
Prussian blue colour
Yellow colour
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers