Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Since aspartame is unstable and decomposes at cooking temperature, therefore, it is used as a sweetening agent in cold foods and soft drinks.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
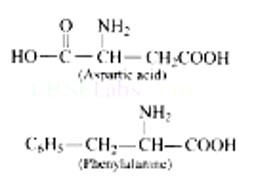
Aspartic acid and phenylalanine are two a-amino acids which form the methyl ester of dipeptide and named as aspartame (an artificial sweetener) which is 100 times more sweet than cane sugar.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Sucralose is trichloro derivative of sucrose. It is about 600 times sweeter than sucrose. However, it neither provides calories nor causes tooth decay.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
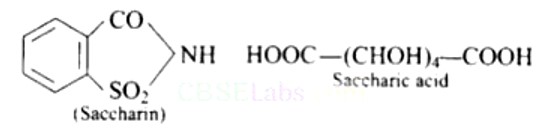
Saccharin (o-sulpho benzoicimide) is an artificial sweetener, whereas saccharic acid (dicarboxylic acid) is obtained by the oxidation of glucose with cone. HNO3 or by bacterial oxidation.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Table salt, sugar vegetable oils and sodium benzoate, etc. are used as preservatives. These do not allow moisture and air to enter the material and hence, bacteria cannot thrive on them. Therefore, pickles do not get spoiled for months.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
In low calorie drinks, some artificial sweetening agents (like aspartame, alitame, sucralose, saccharin etc.) are present which are often many hundred times sweeter than sugar but do not metabolise and hence, do not produce any energy.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Some substances are added to soap to affect the properties in order to make it useful for a particular application, are called fillers. For example, glycerol is added in shaving soaps, to prevent it from rapid drying. Laundry soaps contain fillers like sodium rosinate, sodium silicate, borax and sodium carbonate to increase their leather forming ability. In medicated soaps, substances of medicinal value are added. In some soaps deodorants are also added.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Enzymes have active sites that bind the substrate for effective and quick chemical reaction. The functional groups present at the active site of enzyme react with functional groups of substrate via .ionic bonding, van there Waals' interaction, etc. Some drugs interfere with this interaction by blocking the binding site of enzyme and also prevent the binding of actual substrate with enzyme. This inhibits the catalytic activity of the enzyme. Therefore, these are called inhibitors.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Noradrenaline plays an important role in mood change. If the level of noradrenaline is low, the person suffers from depression.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Histamine is a potent vasodilator. It contracts muscles in the gut and bronchi. It relaxes some other muscles e.g., in the'walls of fine blood vessels. Histamine is also responsible for congestion in the nose associated with common cold and allergies. Histamine stimulates the release of pepsin and hydrochloric acid in the stomach.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers