Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
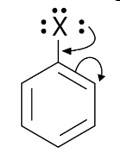
Because electron density is higher at ortho and para locations, the functional groups contained in these compounds are ortho-para directed.
New answer posted
7 months agoContributor-Level 10
The NCERT Exemplar questions go beyond the textbook questions as they are designed to be more challenging and conceptually advanced. Practicing the NCERT exemplar provides a better understanding of complex topics like conductance and electrochemical cells. It also helps in better preparation for the entrance exams. The exemplar often involves multi-concept, application-based, and analytical problems.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Because energy is required to overcome the attractions between the haloalkane molecules as well as to break the hydrogen bonds between water molecules in order to dissolve a haloalkane in water, haloalkanes are only weakly soluble in water. New attractions between the haloalkane and the water molecules, on the other hand, release less energy since they are weaker than the water's initial hydrogen bonds.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
This addition reaction is carried out in accordance with Markovnikoff's rule, which states that in a double bond, the hydrogen from the hydrogen halide is added to the carbon atom with the most hydrogen atoms attached to it, while the halogen atom is attached to the carbon atom with the fewest hydrogens attached to it. The main product in the combination will be the molecule that follows this guideline. As a result, the molecule (B) will be the reaction's main product.
New answer posted
7 months agoContributor-Level 10
A salt bridge in a galvanic cell maintains electrical neutrality by allowing the ions to flow between the two half-cells. It ensures the continuous flow without participating in the reaction. The salt bridge prevents the charges accumulation as such an accumulation can halt the electrochemical reaction by stopping the flow of electrons.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
(a) Br2 gas is produced when NaBr and H2SO4 are combined. Because of the stable molecule created as a result of resonance stabilisation, molecule
(b) Will not react with Br2 gas.
New answer posted
7 months agoContributor-Level 10
The Nernst equation in Electrochemistry is important because, under non-standard conditions, it allows us to calculate the electrode potential of the half and full cell. This equation shows how the potential of the cell varies with the temperature, concentration, and pressure. In electrochemical cells, it helps in predicting the spontaneity and direction of redox reactions.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
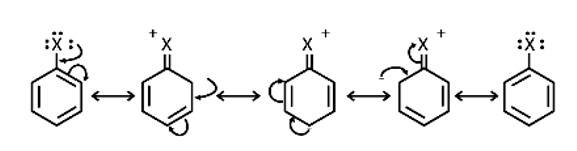
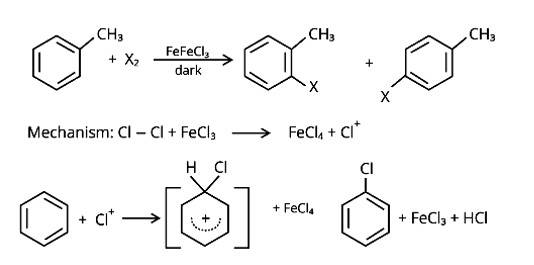
In the presence of Lewis acid catalysts (iron or iron chloride), aryl bromides and chlorides can be made by electrophilic replacement of arenas with bromine and chlorine, respectively.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
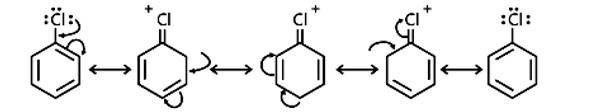
The resonance stabilisation of the aryl ring is the main reason haloarenes are less reactive than haloalkanes and haloalkenes. The electron pairs on the halogen atom, for example, are conjugated with the ring's -electrons in C6H5 - Cl. The C-Cl link takes on a partial double bond character as a result of resonance, making it less reactive to nucleophilic substitution than haloalkanes and haloalkanes.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Triiodomethane is the chemical name for iodoform. Because of its ability to liberate free iodine, it has antibacterial properties.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers