Dual Nature of Radiation and Matter
Get insights from 170 questions on Dual Nature of Radiation and Matter, answered by students, alumni, and experts. You may also ask and answer any question you like about Dual Nature of Radiation and Matter
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
5 months agoNew answer posted
5 months agoContributor-Level 10
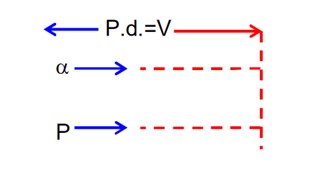
Energy of photon, (so photoelectric effect will take place)
Number of photons falling per second
Number of photoelectron emitted per second
11
New answer posted
5 months agoContributor-Level 10
The light wave contains two lights of different frequencies, so
Maximum kinetic energy of the photoelectron = 5.9-2.5 = 3.42 eV
New answer posted
6 months agoContributor-Level 10
E Energy with which photons are incident on a metallic surface.
E E1 < E2
We know E1 =
k1 =
& E2 =
k2 = (i)
from (1) & (2)
k2 >
New answer posted
6 months agoContributor-Level 10
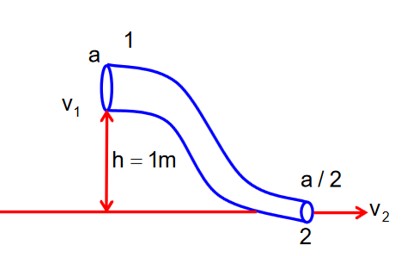
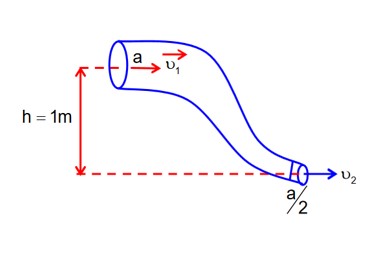
P1 – P2 = 4100 Pa
Bernoulli's question b/w (1) & (2)
-(1)
Equation of continuity
A1v1 = A2v2
v2 = 2v1-(2)
from (1) & (2)
x = 363
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers