Dual Nature of Radiation and Matter
Get insights from 170 questions on Dual Nature of Radiation and Matter, answered by students, alumni, and experts. You may also ask and answer any question you like about Dual Nature of Radiation and Matter
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Electron emission is used for generating a beam of electrons in an electron microscope. An electron microscope requires a source of electrons for creating a beam of electrons. This source can be mostly a gun that uses electron emission for producing free electrons. Once the electrons are emitted, they are accelerated by anode and then, they are focused as a fine beam through a series of electromagnetic lenses. The beam is then directed onto the specimen that is being examined.
New answer posted
5 months agoContributor-Level 10
The work function in electron emission is the minimum amount of energy needed for removing an electron from the surface of a material. This function is denoted as? , and it is measured in electron volts. An electron requires at least the work function as energy to escape the surface of a solid material into the vacuum. This represents the difference between energy of an electron at rest in vacuum and fermi level of the material. Work function varies from material to material.
New answer posted
5 months agoContributor-Level 10
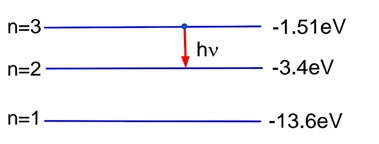
Let the work function of metal is
. (i)
. (ii)
Doing (i) – 3 X (ii), we have
The threshold wavelength =
New answer posted
5 months agoContributor-Level 10
As we know that radius of circular path in magnetic field is given as
= 1.08 eV
New answer posted
5 months agoContributor-Level 10
Schrödinger's wave equation explains the microscopic world that governs chemistry, electronics, and modern technology. It's basically how our digital world actually functions at the atomic level.
New answer posted
5 months agoContributor-Level 10
Computer chips, lasers, MRI machines, LED lights, and solar panels all rely on quantum effects that this equation of Schrodinger helps us understand and design.
New answer posted
5 months agoContributor-Level 10
The Schrodinger wave equation is the foundation of quantum mechanics. Without it, we couldn't understand how atoms work or predict the behaviour of electrons in materials.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers