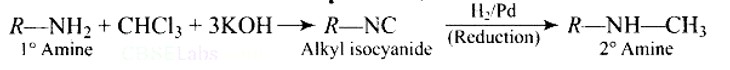Exemplar Solution
Get insights from 1.3k questions on Exemplar Solution, answered by students, alumni, and experts. You may also ask and answer any question you like about Exemplar Solution
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
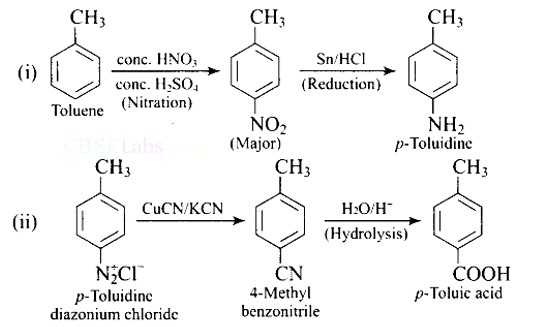
This is a Short Answer Type Questions as classified in NCERT Exemplar
The formation of intramolecular H-bonding between the >C=O groups of amino acids in one turn and the? NH? groups of amino acids in the following turn stabilises a polypeptide chain in the a-helix shape of protein.
New answer posted
8 months agoContributor-Level 10
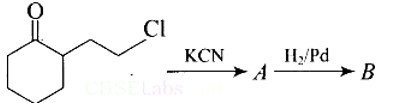
This is a Short Type Questions as classified in NCERT Exemplar
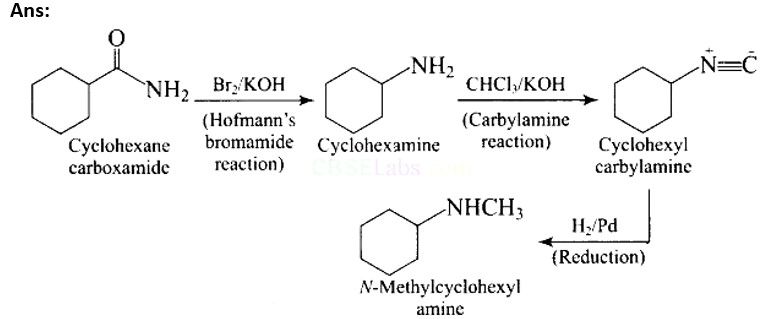
Ans:
New answer posted
8 months agoContributor-Level 10
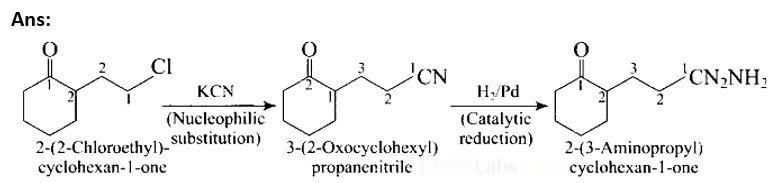
This is a Short Answer Type Questions as classified in NCERT Exemplar
To make a polypeptide chain, the amino acid must be linked to the α− carbon of the molecule. The removal of water molecules from an α− amino acid results in the formation of a polypeptide chain.

New answer posted
8 months agoNew answer posted
8 months agoNew answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Invert sugar is another name for sucrose. Sugar beet sugar is a colourless, crystalline, and sweet substance made from sugar beets. It is very water soluble and has a dextrorotatory aqueous solution [α]D=+66.5? When cane sugar is hydrolyzed with dilute acids or the enzyme invertase, it creates an equimolar mixture of D− (+)− glucose and D− (−)− fructose. Sucrose is dextrorotatory as a result, and hydrolysis produces dextrorotatory glucose and laevorotatory fructose. Fructose's specific rotation is greater than that of glucose. As a result, the ensuing solut
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Aldo-pentoses like ribose and 2 - deoxyribose are sugar moieties found in nucleic acids like RNA and DNA. The structures of these sugars are illustrated below. Both aldopentoses' D- configuration is configuration.
β−D-ribose is mentioned, and its structure is depicted below.
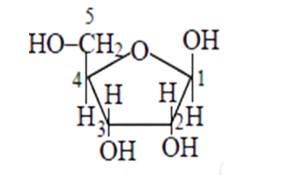
The structure of as β−D-deoxyribose, 2 - deoxyribose is given in the diagram below.
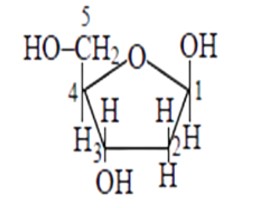
New answer posted
8 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
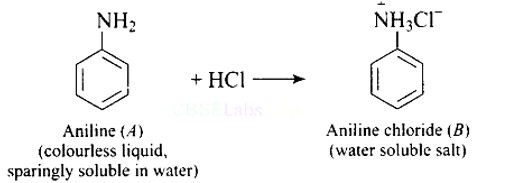
Ans: On reaction with hydrochloric acid, aniline forms anilinium chloride ion which is water soluble. Therefore, the aniline solubility in aqueous HCl solution.
New answer posted
8 months agoContributor-Level 10
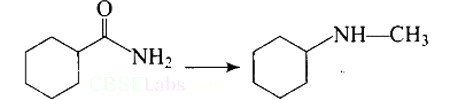
This is a Short Type Questions as classified in NCERT Exemplar
Ans: Carbylamine reaction is shown by primary amines only, primary amines react with chloroform in the presence of alcoholic KOH to form isocyanides which upon catalytic reduction gives secondary amines as the final product.
New answer posted
8 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans: Z is an aliphatic amine that produces a solid that is insoluble in base. This means that the reaction with C6H5SO2Cl must produce a product with no replaceable hydrogen attached to nitrogen.
To put it another way, the amine must be a secondary amine.
Z stands for ethylmethylamine.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers