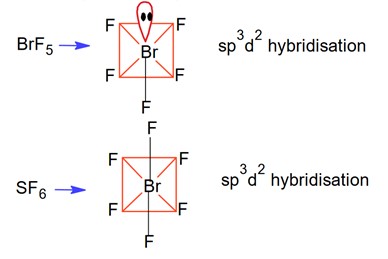
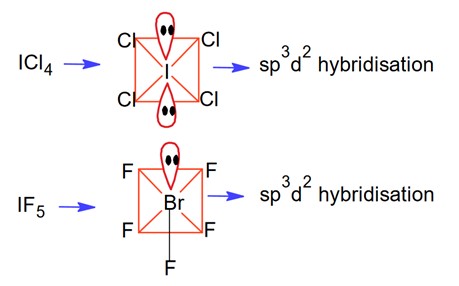
Hybridization
Get insights from 11 questions on Hybridization, answered by students, alumni, and experts. You may also ask and answer any question you like about Hybridization
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoBeginner-Level 5
Xenon has 8 valence electrons. In , it forms two bonds with fluorine, leaving three lone pairs. The steric number =2 bonds +3 lone pairs .
As per the VSEPR Theory the electron? pair geometry due to SN = 5 will be trigonal bipyramidal. The hybridization type will be hybridisation. However, the three lone pairs occupy equatorial positions, resulting in a linear molecular geometry.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers