Hybridization
Get insights from 11 questions on Hybridization, answered by students, alumni, and experts. You may also ask and answer any question you like about Hybridization
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 9
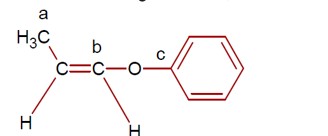
Hybridisation of carbon a, b, and c respectively are sp³, sp² and sp².
New answer posted
5 months agoBeginner-Level 5
The central atom of nitrogen has 5 valence electrons as per the electronic configuraion. During the formation of , 3 valence electrons forms three sigma bonds with hydrogen and one lone electron pair is left.
- The steric number of the ammonia molecule: SN=3 bonds +1 lone pair total electron domains.
- As per the steric number, there is hybridisation in ammonia.
- The lone pair causes repulsion, which leads to a trigonal pyramidal geometry with bond angles of .
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers