Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Liquation refining process is applicable for the metal having low m.p, but containing impurities have higher m.p
New answer posted
3 months agoContributor-Level 10
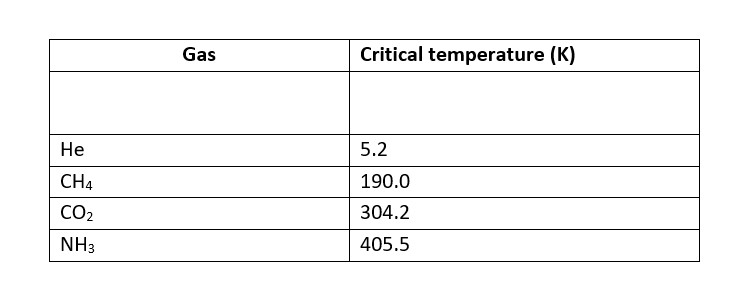
Higher adsorbtion of gas is corresponds to higher liquefaction and higher liquefaction is directly proportional to the higher critical temperature.
New question posted
3 months agoNew answer posted
3 months agoContributor-Level 10
(a) Nitric oxide formation -> Pt is used as catalyst
(b) Haber's process -> Fe is used as catalyst
(c) Hydrolysis of ester -> Acid (H2SO4) is used as catalyst
(d) SO3 formation -> NO is used as catalyst
New answer posted
3 months agoContributor-Level 10
Mole of polyhydric alcohol =
Mole of H2 gas produced =
No of -OH gp present =
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers