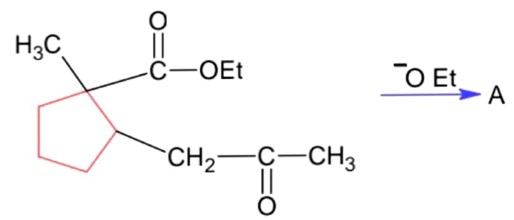
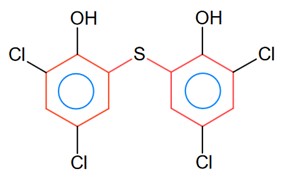
Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
(Calculation is done considering STP condition)
No. of moles ROH = no. of moles of CH4
New answer posted
3 months agoContributor-Level 10
In given electrodes, only is negative
Cr (24) – [Ar] 4s13d54p0
Number of unpaired electrons = 3
New answer posted
3 months agoContributor-Level 10
XeO3 ⇒ Number of lone pair = 1
XeOF4 ⇒ Number of lone pair = 1
XeF6 ⇒ Number of lone pair = 1
New answer posted
3 months agoContributor-Level 10
For single H- atom, maximum number of spectral lines = (n – 1), n = orbit number of excited electron.
New answer posted
3 months agoContributor-Level 10
will not get oxidized, so will not liberate H2 gas upon reaction with acidic solution.
No. of unpaired electron = 4
Spin only magnetic moment =
New answer posted
3 months agoContributor-Level 10
Complex absorbing minimum wavelength of light means the value of for it is maximum.
That complex will be
No. of unpaired electron = 0
New answer posted
3 months agoContributor-Level 10
Mole of polyhydric alcohol =
Mole of H2 gas produced =
No of OH gp present =
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers