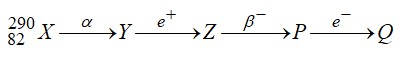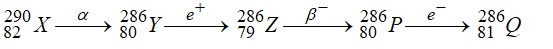Nuclei
Get insights from 103 questions on Nuclei, answered by students, alumni, and experts. You may also ask and answer any question you like about Nuclei
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
N? /N? = e?
For 33% decay, N? /N? = 0.67 ≈ 2/3.
2/3 = e? ⇒ t? = (1/λ)ln (3/2)
For 67% decay, N? /N? = 0.33 ≈ 1/3.
1/3 = e? ⇒ t? = (1/λ)ln (3)
Δt = t? - t? = (1/λ) [ln (3) - ln (3/2)] = (1/λ)ln (2) = T? /? = 20 min
New answer posted
4 months agoContributor-Level 10
C = 200µF, R
Q/A = constant [Given]
A = A? e? & Q = Q? e? /?
Q/A = (Q? /A? ) e^ (-t/RC + λt) = constant
For this to be constant, the exponent must be zero.
-t/RC + λt = 0
1/RC = λ
R = 1/ (λC) =
New answer posted
4 months agoContributor-Level 10
Number of half lives of Y = 3
Number of half lives of X = 6 [As half life of X is half of that of Y].
New answer posted
5 months agoContributor-Level 10
Nuclear force is related to quarks and gluons in the following ways:
- Protons and neutrons are made up of a combination of smaller particles called quarks and down quarks. A proton has two up quarks and one down quark whereas neutron has one up quark and two down quarks.
- Nuclear force is an interaction between quarks that is mediated by particles called gluons. These are the exchange particles that are important for strong interactions. In simple words, they glue quarks together as they are being exchanged between quarks constantly.
- Quarks are never found alone and are often found with protons and neutrons. This happens because of the beha
New answer posted
5 months agoContributor-Level 10
Nuclear force is important to maintain the stability of an atomic nucleus for the following reasons:
- A nucleus has positively charged sub-particles called protons. Since like charges repel each other, a nucleus, in ideal scenario, should fall apart because protons won't be able to coexist in the nucleus. However, nuclear force is extremely strong at very short range. Due to this reason, protons stay bound within nucleus and therefore, nucleus remains stable.
- Nuclear force is responsible for binding energy that holds the nucleons together within a nucleus. Higher binding energy will result in a more stable nucleus.
- The nuclear force shows
New answer posted
5 months agoContributor-Level 10
As we all know that like charges repel each other. This means that there must be a repulsive electromagnetic force between positively charged protons. However, the nuclear force is said to be 100 times stronger than electromagnetic force which makes it strong enough to hold protons together within a nucleus. This nuclear force is much stronger than coulomb force at short ranges.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers