Nuclei
Get insights from 103 questions on Nuclei, answered by students, alumni, and experts. You may also ask and answer any question you like about Nuclei
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
The number of neutrons in nucleus has an important role in determining the stability of nucleus. Stability of nucleus is affected by the balance between number of protons and neutrons and the number of nucleons. In lighter elements, the most stable nuclei has roughly equal number of protons and neutrons. For instance, Carbon-12 is the most common isotope of carbon with 6 protons and 6 neutrons.
New answer posted
5 months agoContributor-Level 10
Most elements exist as a mixture of isotopes in nature. Isotopes are atoms of same elements that have same number of protons but they have different numbers of neutrons. Since the number of neutrons may vary, different isotopes of same element have different atomic masses.
The atomic mass of an element is a weighted average of atomic masses of its isotopes considering their natural abundances. The weighted average is calculated by multiplying atomic mass of every isotope by its natural abundance and then adding these products.
The atomic mass is an average that accounts for different masses and abundances of isotopes that results in a fr
New answer posted
5 months agoContributor-Level 10
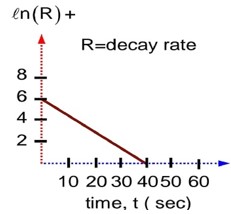
t1/2 = 2hr 30 min
Radiation intensity a disintegrations / sec (Activity)
As,
For safe working,
= 15
New answer posted
5 months agoContributor-Level 10
Given A2 =
Bernoulli's b/w (1) & (2)
[z1 = z2]
[Q = A1V1 = A2V2]
= 2.4 * 10-2 m3 /sec
= 24 * 10-3 m3 /sec
= 24
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers