Nuclei
Get insights from 103 questions on Nuclei, answered by students, alumni, and experts. You may also ask and answer any question you like about Nuclei
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Explanation- dN/dt=
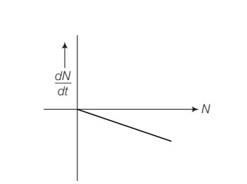
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Explanation- nuclei 2He4 and 1He3 have the same mass number . the element having more number of proton having more repusion so less binding energy. So 2He4 have more number of proton so more repulsion so less binding energy.
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation- B.E = (Mp+MH-MN)c2
B.E= (118.9058+1.0078252-119.902199)c2
B.E=0.0114362 c2
B.E= (Mp+MH-MN)c2
B.E= (119.902199+1.0078252-120.902822)c2
B.E= 0.0059912c2
(ii) the existence of magic numbers indicates that the shell structure of nucleus is similar to the shell structure of an atom. This also explains peaks in binding energy curve.
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation-
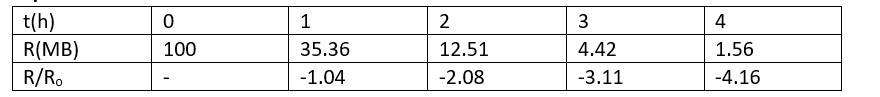
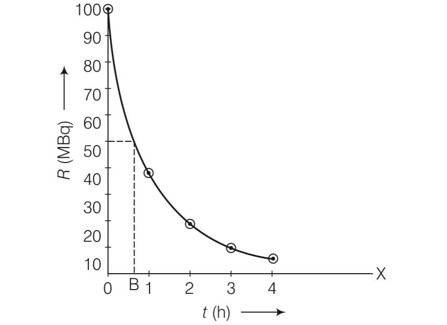
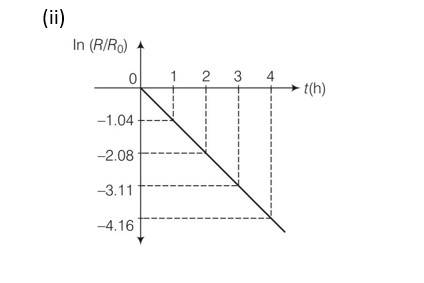
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation- Pn=Pp+Pe
Pp+Pe=0
Pe=Pp=P
Ep= 1/2
Ee= 1/2
= 1/2
From conservation of energy
1/2= 1/2= mnc2
mpc2= 936MeV, mnc2=938MeV, mec2=0.51MeV
since the energy difference n and p is small
mpc2+
pc= mnc2-mpc2 = 938-936= 2MeV
Ep=( )1/2=
= 2.06MeV
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation-E=
If proton and neutron had charge e each and were governed by the same electrostatic force, then in the above equation we would need relace m
So m' = =M/2=918m
Hence binding energy= 918me'4/8
Dividing eqn
918 ( 4= =
= 3.64
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation- B.E= 2.2MeV
E-B=Kn+KP=
Conservation of momentum= Pn+PP= E/C
E=B
It only happen if Pn=Pp=0
Let E=B+X where X<
X= (E/C-PP)/2m+
2 + =0
Pp=
= 4E2/c2
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation- let 38S have N1 active nuclei and 38Cl have N2 active nuclei
+
N1=N0
+
Multiplying by and then integrating both sides we got
N2=
After solving it we get time t= (log )/
t= =
New answer posted
8 months agoContributor-Level 10
13.31 Amount of Electric power to be generated, P = 200000 MW
10% of which to be obtained from nuclear power.
Hence, amount of nuclear power = 10% of 2 MW = 2 MW = 2 J/s
= 2 J/y = 6.3072 J/y
Heat energy release per fission of a nucleus, E = 200 MeV
Efficiency of a reactor = 25%
So the amount of electrical energy converted from heat energy per fission = 25% of 200 MeV = 50 MeV
= 50 eV = 50 J = 8 J
Therefore, number of atoms required per year = = 7.884
1 mole of = 235 gm of contains 6.023 atoms
Hence the mass of 7.884 = 7.884 = 30.76 kg
Hence, the Urani
New answer posted
8 months agoContributor-Level 10
13.30 Amount of hydrogen, m = 1 kg = 1000 g
1 mole of hydrogen, i.e. 1 g of hydrogen ( contains 6.023 atoms
1 kg of hydrogen contains = 1000 6.023 atoms = 6.023 atoms
Within Sun, four ( nuclei combine and forms 1 nucleus. In this process 26 MeV of energy is released.
Hence the energy released from fusion of 1 kg of is:
= MeV = 3.91495 MeV
Amount of , m = 1 kg = 1000 g
1 mole of , i.e. 235 g of contains 6.023 atoms
1 kg of contains = 6.023 atoms = 2.563 atoms
It is known that the amount of energy released in the fission of 1 atom of is 200
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
