Nuclei
Get insights from 103 questions on Nuclei, answered by students, alumni, and experts. You may also ask and answer any question you like about Nuclei
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
13.6 In - decay, there is a loss of 2 protons and 4 neutrons. In every decay, there is a loss of 1 proton and a neutrino is emitted from the nucleus. In every decay, there is a gain of 1 proton and an antineutrino is emitted from the nucleus.
+
+
+ +
+ +
+ +
+ +
+
New answer posted
6 months agoContributor-Level 10
13.1 Mass of lithium isotope, = 6.01512 u
Mass of lithium isotope, = 7.01600 u
Abundance of , = 7.5%
Abundance of , = 92.5%
The atomic mass of lithium atom is given as:
m = = = 6.940934 u
Mass of Boron isotope, = 10.01294 u
Mass of Boron isotope, = 11.00931 u
Let the abundance of be x % and that of be (100-x) %
The atomic mass of Boron atom is given as :
10.8111 =
1081.11 = 1100.931 - 0.99637x
x = 19.89 %
Hence the abundance of is 19.89 % and that of &nb
New answer posted
7 months agoContributor-Level 10
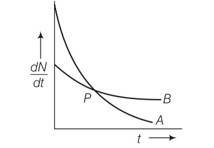
This is a Multiple Choice Questions as classified in NCERT Exemplar
Answer- (c, d)
Explanation- in given figure the slope of curve A is greater than slope B. so decay in A is faster than B . also wavelength of A is grater than B.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Answer- (b, d)
Explanation- this is only possible when rate of decay of 1 is almost double the rate of decay of other.so in this case initial decay of A must be twice of B. also Initial rate of decay of B is same as the rate of decay of A at t = 2h and λA < B
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Answer- (a, b)
Explanation- fusion process are almost impossible at ordinary temperature because nuclei are positive charge and forces between them is of short range.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Answer- (b)
Explanation- the moderator used have light nuclei . because when we use heavy nuclei then there will be collision of proton which does not slow down the process.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Answer- (b)
Explanation – a nucleus contain more number of neutron than protons because the repulsive force is more if it contain more proton and become more unstable.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Answer- (a)
Explanation – tritium contain 1 proton and 2 neutron but when it disintegrates and remove 1 beta particle it will now contain 2 proton and 1 neutron so it will change into 2He3 but its energy will be less from it.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Answer –a
Explanation- + decay represents
zXA z-1YA+1eo+v+Q2
Q2= [mn (zXA)-mn (z-1YA)-me]c2
= (Mx-My-2me)c2
- decay represents
zXA z+1YA+-1eo+v+ 1
1= [mn (zXA)-mn (z+1YA)-me]c2
= (Mx-My)c2
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Answer- (b)
Explanation – because alpha and beta particle have some charge and mass so their energy level will change but in case of gamma particle it does not contain any charge.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers