Organic Chemistry - Some Basic Principles and Tech
Get insights from 110 questions on Organic Chemistry - Some Basic Principles and Tech, answered by students, alumni, and experts. You may also ask and answer any question you like about Organic Chemistry - Some Basic Principles and Tech
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
Moles of chlorine in the given compound = Moles of chlorine in AgCl
= moles of AgCl
Mass of chlorine =
= 0.098 g
New answer posted
8 months agoContributor-Level 10
The resonance structures have
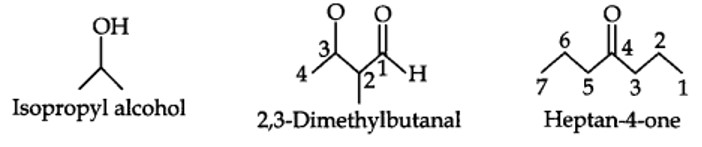
(i) The same positions of nuclei, and
(ii) The same number ofunpaired electrons.
New answer posted
8 months agoContributor-Level 10
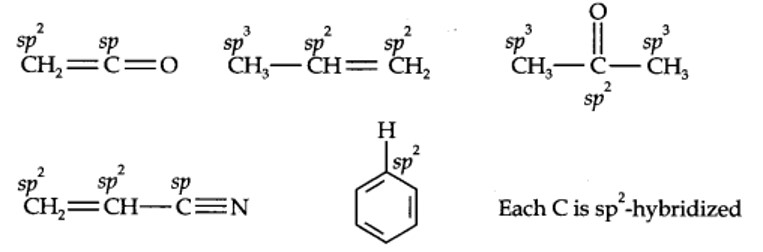
The resonance effect is defined as 'the polarity produced in the molecule by the interaction of two π -bonds or between a π -bond and lone pair of electrons present on an adjacent atom'. The effect is transmitted through the chain. There are two types of resonance or mesomeric effect designated as +R and-R effect.
The atoms or substituent groups, whichrepresent +R or –R electron displacementeffects are as follows:
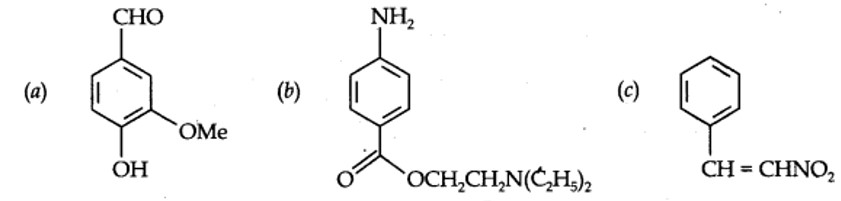
+R effect: – halogen, –OH, –OR, –OCOR, –NH2, –NHR, –NR2, –NHCOR,
– R effect: – COOH, –CHO, >C=O, – CN, –NO2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers