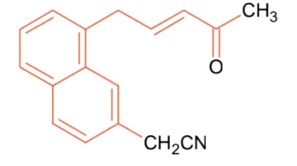
Organic Chemistry - Some Basic Principles and Tech
Get insights from 110 questions on Organic Chemistry - Some Basic Principles and Tech, answered by students, alumni, and experts. You may also ask and answer any question you like about Organic Chemistry - Some Basic Principles and Tech
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
Meq of NH3 = Meq of used H2SO4 = Meq of NaOH = 0.25 * 30 = 7.5
Millmoles of N = millimoles of NH3 = 7.5 (As n factor = 1)
Mass of nitrogen = 7.5 * 14 * 10-3 = 0.105 gm
% of Nitrogen =
New answer posted
6 months agoContributor-Level 10
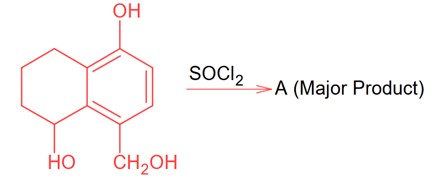
Since SOCl2 is used to covert aliphatic (R-OH) into chlorides. It will not react with aromatic alcohol
New answer posted
6 months agoContributor-Level 10
Organic compound → AgBr (s)
0.5 g 0.40 g
Here; Moles of Br in organic compound = Moles of Br in Ag Br
= Moles of AgBr
=
Mass of Br in organic compound =
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers

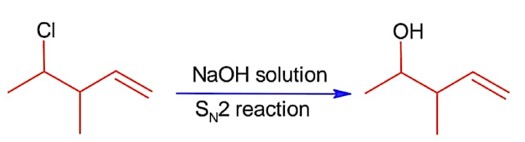 ffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffff
ffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffff