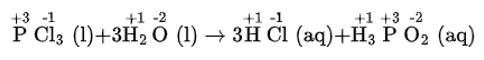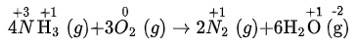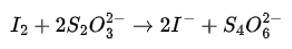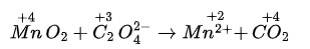Redox Reactions
Get insights from 164 questions on Redox Reactions, answered by students, alumni, and experts. You may also ask and answer any question you like about Redox Reactions
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (iv) is the correct answer
The strongest oxidising agent means it has greater tendency to oxidise other species and itself gets easily reduced. So higher the E? values, stronger is the oxidising agent it is. Thus, Ag+ having the highest positive E? value among the given systems, is the strongest oxidising agent.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
option (iv) is the correct answer.
Redox reaction is defined as the simultaneous oxidation and reduction of reacting species. Thus, change in oxidation state will decide whether a reaction is redox or not. Thus, assigning the oxidation states as:
(i) CuO + H2→ Cu + H2O
Here, oxidation of H and reduction of Cu is taking place.
(ii) Fe2O3 + 3CO→ 2Fe + 3CO2
Here, oxidation of C and reduction of Fe is taking place.
(iii) 2K + F2→2KF
Here, the oxidation of K and reduction of F is taking place.
(iv) BaCl2 + H
New answer posted
7 months agoContributor-Level 10
This is a Short answer type question as classified in NCERT Exemplar
(i) The balanced chemical is given as –
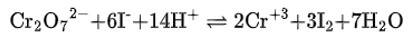
(ii) The balanced chemical is given as –
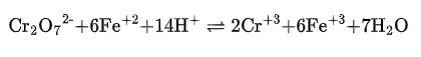
(iii) The balanced chemical is given as –
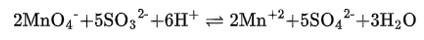
(iv) The balanced chemical is given as –
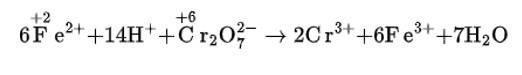
New answer posted
7 months agoContributor-Level 10
(i) We can write the given reaction along with their oxidation numbers as-
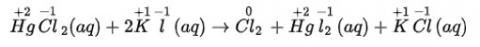
As, chlorine is oxidised in hydrochloric acid (behaving as reducing agent) -as its oxidation number is increases during the reaction from -1 to 0) and nitric acid is reduced (behaving as oxidising agent) as its oxidation number is decreases during the reaction from +5 to +3). Hence, it is the redox reaction.
(ii) We can write the given reaction along with their oxidation numbers as-
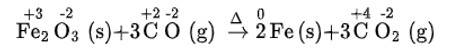
Here, oxidation number of none of the atoms change hence it is not a redox reaction.
(iii) We can write
New answer posted
7 months agoContributor-Level 10
This is a Short answer type question as classified in NCERT Exemplar
(i) We can balance the given equation by oxidation number method-
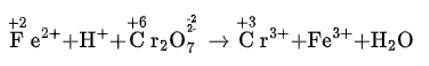
(a)Balance the increase and decrease in O.N.
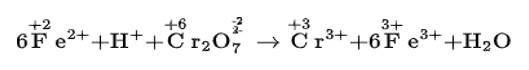
(b) Balancing H and O atoms by adding H+ and H2O molecules
(ii) We can balance the given equation by oxidation number method-
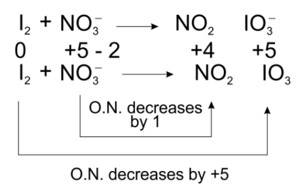
Total decrease in O.N. = 1
To equilize O.N. multiply NO3-, by 10
I2 + 10 NO3-? 10NO2 + IO3-
Balancing atoms other than O and H
I2 + 10 NO3-? 10NO2 + 2 IO3-
Balancing O and H
I2 + 10 NO3- + 8H+? 10NO2 + 2 IO3- + 4H2O
(iii) We can balance the given equation by oxidation number method
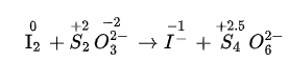
Total increase in O.N. =
New answer posted
7 months agoContributor-Level 10
(a) let x= oxidation number of sulphur, and +1 is oxidation number of Na, -2 is oxidation number of O, also we can assume total charge on compound = 0 then solving we get.
+2 + 2x - 6 = 0
x = +2.
(b) let x= oxidation number of sulphur, and +1 is oxidation number of Na, -2 is oxidation number of O, also we can assume total charge on compound = 0 then solving we get.
+2 + x - 6 = 0
x = +4
(c) let x= oxidation number of sulphur, and +1 is oxidation number of Na, -2 is oxidation number of O, also we can assume
New answer posted
7 months agoContributor-Level 10
This is a Short answer type question as classified in NCERT Exemplar
(i) Let the oxidation number of P in HPO32- be x.
Therefore, +1 + x + (-6) = -2
x = +3
Oxidation number of phosphorus is= +3.
(ii) Let the oxidation number of P in PO43- be x.
Therefore, x + (-8) = -3
x = +5
Oxidation number of phosphorus is= +5.
New answer posted
7 months agoContributor-Level 10
We can balance the given reaction by ion electron method as
Cl2O7 (g) + H2O2 (aq) → ClO2- +O2 (acidic medium)
This is a Short answer type question as classified in NCERT Exemplar
Balancing bu ion electron method
2 * { Cl2O7 + 6H+ + 8e- → 2ClO2- + 3H2O
8 * { H2O2 → O2 + 2H+ + 2e-
The balanced chemical is given as-
2Cl2O7 + 12H+ + 16e- → 4ClO2- + 6H2O
8H2O2 → 8O2 + 16H+ + 16e-
2Cl2O7 + 8H2O2 → 4ClO2- + 6H2O + 8O2 + 4
New answer posted
7 months agoContributor-Level 10
This is a Short answer type question as classified in NCERT Exemplar
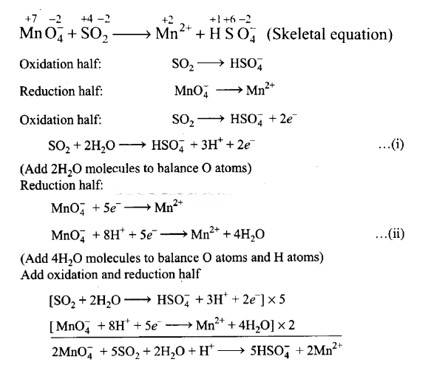
(i) 2Mn0–4 + 5S02 + 2H20 + H+?5HS0–4 + 2Mn2+
Balancing by ion-electron method:
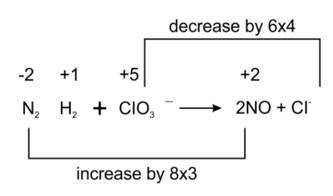
(ii) We can balance the given reaction by oxidation number method-
Balancing by oxidation number method as to make the electron gain and loss equal as given
3N2H4 + 4ClO3- → 6NO + 4Cl- + 6H2O
The balanced chemical is given as-
(iii) We can balance the given reaction by ion electron method as
Cl2O7(g) + H2O2 (aq) → ClO2- +O2 (acidic medium)
Balancing bu ion electron method
2 * { Cl2O7 + 6H+ + 8e- → 2ClO2- + 3H2O
8 * { H2O2 → O2 + 2H+ + 2e-
The balanced chemical is
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers