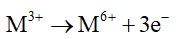Redox Reactions
Get insights from 164 questions on Redox Reactions, answered by students, alumni, and experts. You may also ask and answer any question you like about Redox Reactions
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
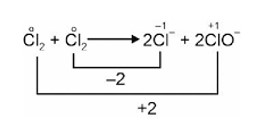
After balancing change in oxidation state,
2Cl2 2Cl– + 2ClO–
Next, balance 'O' atoms,
2Cl2 +4OH–
Simplifying to get the simplest ratios,
Cl2 +2OH–
x = 1, y = 2, z = 1, p = 1
New answer posted
3 months agoContributor-Level 10
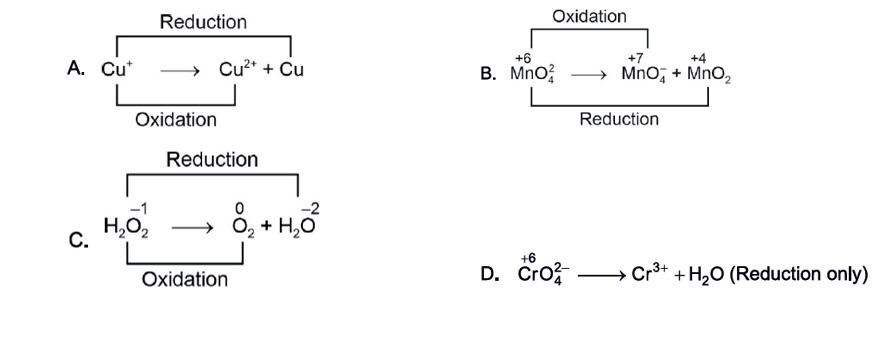
Disproportionation reaction is a reaction in which a substance (element) is simultaneously oxidised and reduced.
New answer posted
3 months agoContributor-Level 10
Potassium hydrogen phthalate is used to standardize NaOH solutions.
Phenolphthalein is used as an indicator to detect completion of titrations.
New answer posted
3 months agoContributor-Level 10
Oxidation no. of a substance is proportional to the charge on it. In this case the charge is +
New answer posted
3 months agoContributor-Level 10
In NEET exam, there can be 1 to 2 questions from redox reactions, carrying 4-8 marks. The weightage is around 2% to 4%. In JEE Main, the weightage of this chapter is from 3.3% to 4-6%. You can expect nearly 1 to 2 questions from this chapter.
New answer posted
3 months agoContributor-Level 10
The applications of redox reactions include in cellular respiration, and batteries. In industries, these are used in the chemical production, metal extraction and water treatment.
New answer posted
3 months agoContributor-Level 10
The redox reactions are significant for some of the basic functions of life such as respiration, photosynthesis, corrosion or rusting and combustion.
Related Tags
New answer posted
3 months agoContributor-Level 10
There are four types of redox reactions. These are combination, Decomposition, Displacement, and Disproportionation.
In the combination redox reaction, two or more substances form a single product, in decomposition reactions, a compound breaks down into two or more substances. When one element in a compound replaces another, it is called displacement reactions. When simultaneously, one substance is oxidized and reduced, the reaction is known as the disproportionation redox reaction.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers