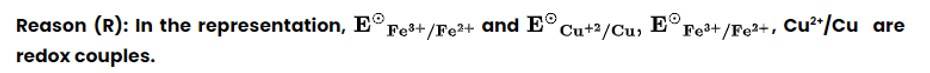Redox Reactions
Get insights from 164 questions on Redox Reactions, answered by students, alumni, and experts. You may also ask and answer any question you like about Redox Reactions
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Option (ii) is the correct answer.
A redox couple is defined as pair of compounds or elements having together the oxidised and reduced forms of it and taking part in an oxidation or reduction half reaction.
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Option (i) is the correct answer.
Here the oxygen of peroxide, which is present in -1 state, is converted to zero oxidation state in O2 undergoing oxidation and decreases to -2 oxidation state in H2O undergoing reduction
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Option (iii) is the correct answer
As permanganate ion changes to MnO2
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Option (i) is the correct answer.
Fluorine is most electronegative element that is why it is best oxidant among halogens
New answer posted
7 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
(i) → (e); (ii) → (d); (iii) → (c); (iv) → (b); (v) → (f).
New answer posted
7 months agoContributor-Level 10
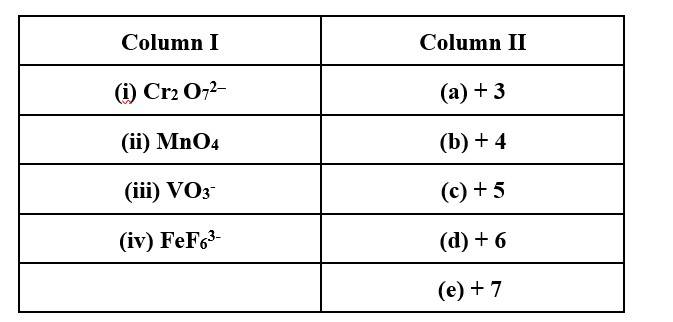
This is a Matching Type Questions as classified in NCERT Exemplar
(i) → (d); (ii) → (e); (iii) → (c); (iv) → (a)
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (i) and (ii) are the correct answers.
The ones which will act as anodes when connected to standard hydrogen electrode as they have more negative standard reduction potential as compared to standard hydrogen electrode. The one which will act as cathodes when connected to standard hydrogen electrode as they have more positive standard reduction potential as compared to standard hydrogen electroDE
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Hence, option (iii) and (iv) are the correct answers.
The given reaction is as below-
P4+3OH- +3H2O→PH3 + 3H2PO2-
The above reaction is a kind of disproportionate reaction in which phosphorous is being reduced as well as oxidized whereas hydrogen remains same in +1 oxidation state.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (ii), (iii) and (iv) are the correct answers.
Elements that are having only s-electrons in the valence shell do not show more than one oxidation state (shows only one oxidation state of +1). Hence, (b), (c) having incompletely filled d-orbital's in the outermost shell show variable oxidation states. Element with outer electronic configuration as 3d1 4S2 shows variable oxidation states of +2 and +3 and the element with outer electronic configuration as 3d24S2 shows variable oxidation states of +2, +3 and +4. P-Block elements also show va
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (iii) and (iv) is the correct answer.
The given equation is as-
Zn+2HCl→ZnCl2+H2
In this Zn has a negative E? value, which means it will undergo oxidation and will act as a reducing agent (reductant). Zn can produce H2 gas with HCl, as hydrogen has higher standard reduction potential than Zn, hydrogen will undergo reduction and will act as oxidant.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers