States of Matter
Get insights from 60 questions on States of Matter, answered by students, alumni, and experts. You may also ask and answer any question you like about States of Matter
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
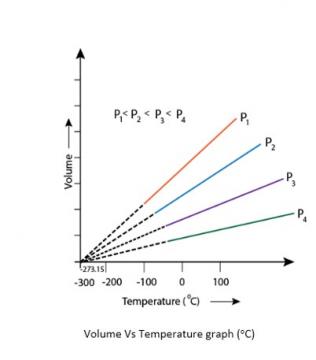
5.21. At -273°C, volume of the gas becomes equal to zero, i.e., the gas ceases to exist.
New answer posted
8 months agoContributor-Level 10
5.20. SI unit of pressure, P = Nm-2
SI unit of volume = m3
Si unit of temperature, T = K
SI unit of number of moles, n = mol
Thus, SI unit of pV2T2/n = (Nm-2) (m3)2 (K) 2mol = Nm4K2mol-1
New answer posted
8 months agoContributor-Level 10
5.19. As the mixture H2 and O2 contains 20% by weight of dihydrogen, therefore,
If H2 = 20g, then O2 = 80g
No. of moles of H2 = 20/2 = 10 moles
No. of moles of O2 = 80/32 = 2.5 moles
Partial pressure of H2 = [No. of moles of H2/ (No. of moles of H2 + No. of moles of O2)] x
Ptotal= [10 (10 + 2.5)] x 1 = 0.8 bar
New answer posted
8 months agoContributor-Level 10
5.18. Given, P1 = P2 and V1 = V2
We know that P1V1 = P2V2
Or, n1RT1 = n2RT2
i.e., n1T1 = n2T2
Substituting n = w/M, we get
(W1/M1) x T1 = (W2/M2) x T2
(2.9/M1) x (95 + 273) = (0.184/2) x (17 + 273)
M1 = (2.9 x 368 x 2) / (0.184 x 290) = 40 g mol-1
New answer posted
8 months agoContributor-Level 10
5.17. No. of moles of CO2 = Given mass of CO2 / Molar mass
= 8.8g / 44g mol-1 = 0.2 mol
Pressure of CO2 = 1 bar
RR = 0.083 bar dm3 K–1 mol–1
T = 273 + 31.1 K = 304.1 K
According to ideal gas equation,
PV = nRT
Therefore, V = nRT/P
= (0.2 x 0.083 x 304.1) / 1 bar = 5.048 L
New answer posted
8 months agoContributor-Level 10
5.16. Radius of the balloon = 10 m
Therefore, volume of the balloon = (4/3)? r3 = (4/3) x (22/7) x (10 m)3
= 4190.5 m3
Volume of He filled at 1.66 bar and 27 °C = 4290.5 m3
To calculate the mass of He,
PV = nRT = (w/M) RT, where M is molar mass of He i.e. 4 g per mole or 4 x 10-3 kg mol-1
=> w = [ (4 x 10-3 kg mol-1) (1.66 bar) (4190.5 m3)] / [ (0.083 bar dm3 K-1 mol-1) (300K)]
= 1117.5 kg
Total mass of the balloon along with He = 100 + 1117.5 = 1217.5 kg
Maximum mass of the air that can be displaced by balloon to go up = volume x density
= 4190.5 m3 x 1.2 kg m-3 = 5028.6 kg
New answer posted
8 months agoContributor-Level 10
5.15. Molar mass of O2 = 32 g/mol
It means, 8 g of O2 has 8/32 mol = 0.25 mol
Molar mass of H2 = 2 g/mol
It means, 4 g of H2 has 4/2 mol = 2 mol
Therefore, total number of moles, n = 2 + 0.25 = 2.25 mol
Given, V = 1dm3, T = 27°C = 300 K, R = 0.083 bar dm3 K-1 mol-1
Applying PV = nRT,
P = nRT / V
= (2.25) (0.083 bar dm3 K-1 mol-1) (300 K) / (1dm3)
= 56.025 bar
New answer posted
8 months agoContributor-Level 10
5.14. Time taken to distribute 1010 wheat grains = 1s
Time taken to distribute Avogadro number of wheat grains = (1s x 6.022 x 1023) / 1010
= 6.022 x 1013 s
= (6.022 x 1013 / 60 x 60 x 24 x 365) year
= 1.9 x 106 years
New answer posted
8 months agoContributor-Level 10
5.13. Molecular mass of N2 = 28g
28 g of N2 has No. of molecules = 6.022 x 1023
1.4 g of N2 has No. of molecules = (6.022 x 1023 x 1.4 g)/28 g= 3.011 x 1022 molecules.
Atomic No. of Nitrogen (N) = 7
1 molecule of N2 has electrons = 7 x 2 = 14
3.011 x 1022 molecules of N2 have electrons= 14 x 3.011 x 1022= 4.215 x1023 electrons.
New answer posted
8 months agoContributor-Level 10
5.12. Given,
P= 3.32 bar
V= 5 dm3
n= 4 mol
R= 0.083 bar dm3 K-1 mol-1
PV = nRT
Or T = PV / nR = 3.32 x 5 dm3 / (4.0 mol x 0.083 bar dm3 K-1 mol-1)
= 50 K
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
