States of Matter
Get insights from 60 questions on States of Matter, answered by students, alumni, and experts. You may also ask and answer any question you like about States of Matter
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
F = -dU/dr = - [-12A/r¹³ + 6B/r? ]
F=0 ⇒ r= (2A/B)¹/?
U (at r= (2B/A)¹/? ) = -A²/4B
New answer posted
5 months agoContributor-Level 10
After removal A = 4
After removal B = 4 – 1 = 4 (only two atoms are removed)
Final formula of the compound = A3B4
New answer posted
5 months agoContributor-Level 10
PV = nmixRT
Let moles of He is x
Moles of H2 = 3 – x
4x + 2 (3 – x) = 10
x = 2 mol
Mass of He = 8 gm
New answer posted
6 months agoContributor-Level 10
For ideal gas using ;
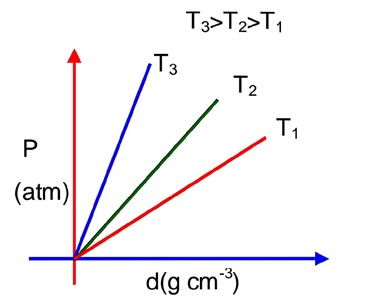
PM = dRT
Comparing with y = mx + C
Slope, m =
Slope
So; higher the slope higher the T
Hence, T3 > T2 > T1
New question posted
6 months agoRelated Tags
New answer posted
8 months agoContributor-Level 10
5.42. Dalton's law of partial pressure: When two or more non-reacting gases are enclosed in a vessel, the total pressure of the gaseous mixture is equal to the sum of the partial pressures that each gas will exert when enclosed separately in the same vessel at constant temperature.
P= P1 + P2 + P3
Where, P is the total pressure of the three gases A, B, and C enclosed in a container. P1, P2 and P3 are the partial pressures of the three gases when enclosed separately in the same vessel at a given temperature one by one.
No, the law cannot be applied. Carbon monoxide and oxygen readily combine to form carbon dioxide. The law can be appl
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
