Surface Chemistry
Get insights from 216 questions on Surface Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Surface Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
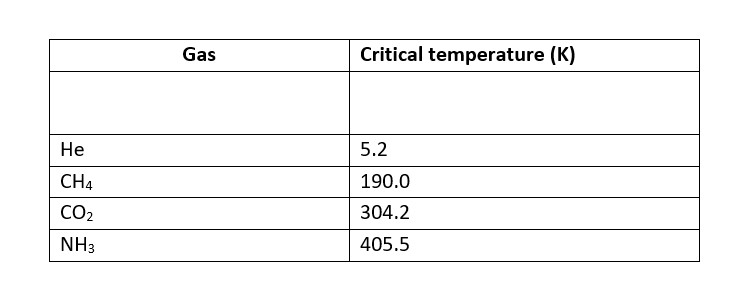
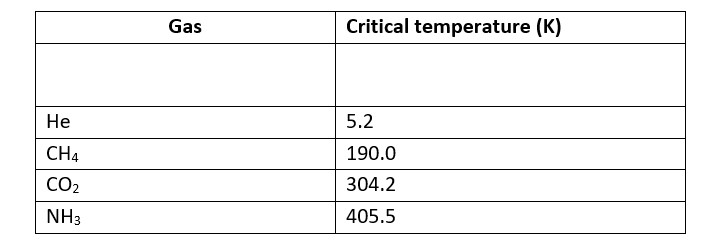
Higher adsorbtion of gas is corresponds to higher liquefaction and higher liquefaction is directly proportional to the higher critical temperature.
New answer posted
6 months agoContributor-Level 10
During micelle formation,
Micelle formation is endothermic at low temperature and exothermic at high temperature.
At room temperature ⇒ Micelle formation is endothermic.
New answer posted
6 months agoContributor-Level 10
Emulsion are liquid colloids, protective colloids are lyophilic, FeCl3 + H2O charged colloids and FeCl3 + NaOH (-ve)ly charged colloids.
New answer posted
6 months agoContributor-Level 10
SO2 is absorbed to a greater extent then CH4 on activated charcoal under same Gases with higher critical temperature are readily absorbed by activated charcoal.
New answer posted
6 months agoContributor-Level 10
Always fresh solution of starch used and recorded immediately after the blue colour appears. Na2S2O3 is used in limited amount.
New answer posted
6 months agoContributor-Level 10
Higher adsorbtion of gas is corresponds to higher liquefaction and higher liquefaction is directly proportional to the higher critical temperature.
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
→ number of electrons = 10
Br → number of electrons = 36
→ number of electrons = 2
→ number of electrons = 18
→ number of electrons = 2
→ number of electrons = 18
K+ → number of electrons = 18
O-2 → number of electrons = 10
Mg+2 → number of electrons = 10
O-2 & Mg+2 are isoelectronic with Al+3
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers