Alcohol Phenol And Ethers
Get insights from 261 questions on Alcohol Phenol And Ethers, answered by students, alumni, and experts. You may also ask and answer any question you like about Alcohol Phenol And Ethers
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
8 months agoNew question posted
8 months agoNew answer posted
8 months agoContributor-Level 10
11.47
(a) (i) Primary alcohols do no react appreciably with Lucas' reagent (HCl –ZnCl2) at room temperature.
(ii) Tertiary alcohol reacts immediately with Lucas 'reagent.
New answer posted
8 months agoContributor-Level 10
11.46 In this reaction, when propene reacts with the given reagent then the double bond of propene breaks down with charges on them. So, H+ gets placed on the carbon which already has two hydrogen atom and OH- gets substituted on center carbon because it has the more positive charge which attracts OH-. Thus we get propene-2-or as a
- In this reaction, when Methyl ( 2-oxocyclohexyl) ethanoate reacts with the given reagent then the double bond between the oxygen atom and cyclohexyl gets breaks down, such that O has a negative charge and that particular carbon will have a positive charge on it. So, to neutralize it, H+ gets substituted
New answer posted
8 months agoContributor-Level 10
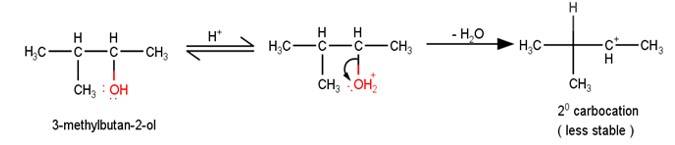 A
A
The next step is a rearrangement of the 20 carbocations formed in the above step is less stable it rearranges by a 1,2-hydride shift to form more stable 3° carbocations.
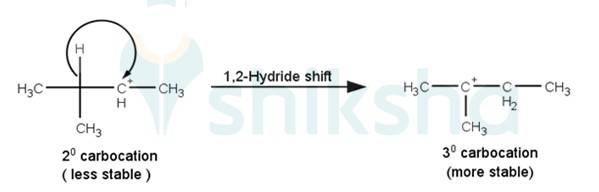
The last step of the reaction is the nucleophilic attack of Br- ion on the 3° carbocations giving the final product.
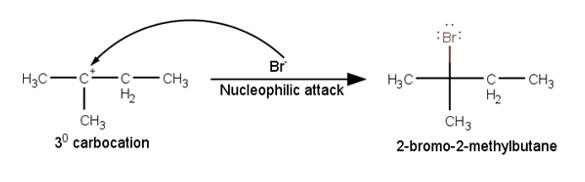
New question posted
8 months agoNew answer posted
8 months agoContributor-Level 10
11.43
The driving force of all the reactions given to the question is that the alkoxy group is an ortho and para directing group because it exerts its +R effect in the benzene ring. Para position being comparatively more stable than the ortho position is usually preferred because ortho position leads to stearic hindrance, hence the major product is mostly the para- substituted compound.
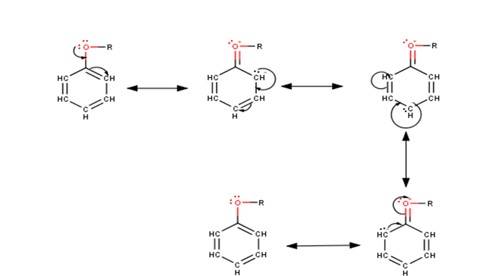
As seen from the resonating structures above the structure in which the negative charge is in the para position will form a more stable product when attacked by an electrophile. Hence in the following reactions, we will be considering that resonating str
New answer posted
8 months agoContributor-Level 10
11.42
The reaction of HI with methoxymethane yields two different sets of products depending upon the initial amount of HI taken.
(i) When equal moles of HI and methoxymethane are taken, a mixture of methyl alcohol and methyl iodide is
The mechanism is given below:
In the first step, methoxymethane reacts with hydrogen iodide to extract a proton to give the dimethyloxonium ion.
In the second step of the reaction, the Dimethyloxonium ion reacts with the iodide ion present to yield methyl iodide and methyl alcohol as the product via SN2 pathway.
(ii) If an excess of HI is used the methyl alcohol formed in Step II is also convert
New answer posted
8 months agoContributor-Level 10
11.41
(i) In aryl alkyl ethers the +R effect of the alkoxy group leads to an increase in the electron density of the benzene ring as they push electrons into the ring making the benzene ring activated towards electrophilic substitution reactions. This could be understood more clearly from the following resonating structures : -
(ii) It could be clearly seen from the above resonating structures that the electron density increases more at the ortho and para positions as compared to the meta positions. Hence, we can conclude that the alkoxy group directs the incoming substituents to ortho and para positions in the benzene ring.
For e
New answer posted
8 months agoContributor-Level 10
11.40
1-Propoxypropane reacts with hydrogen iodide to give propan-1-ol and 1-iodopropane as the products.
2. Methoxybenzene reacts with hydrogen iodide to give phenol and iodomethane.
3. Benzyl ethyl ether reacts with hydrogen iodide to give benzyl iodide and ethanol.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers

