Alcohol Phenol And Ethers
Get insights from 261 questions on Alcohol Phenol And Ethers, answered by students, alumni, and experts. You may also ask and answer any question you like about Alcohol Phenol And Ethers
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
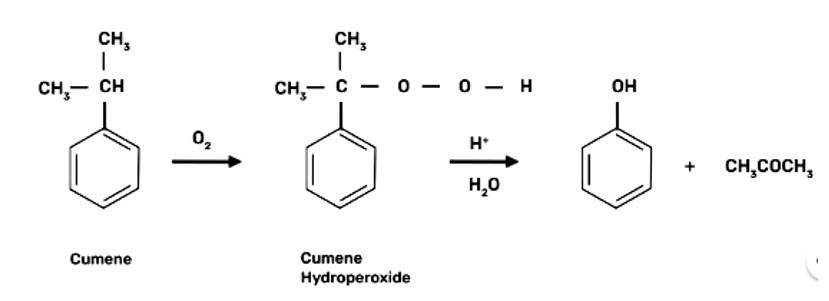
(a) Cumene is the beginning element for the industrial production of phenol.
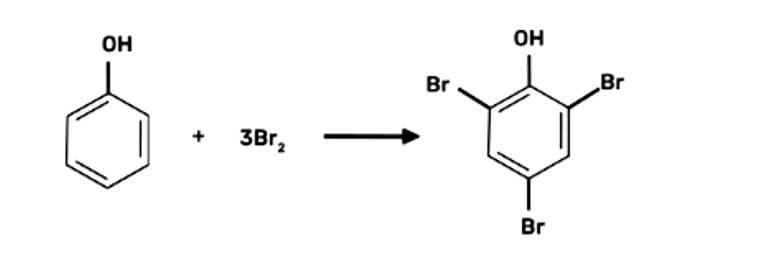
When bromine water is used to treat phenol. As a whitish precipitate, 2,4,6-tribromophenol is formed
New answer posted
8 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
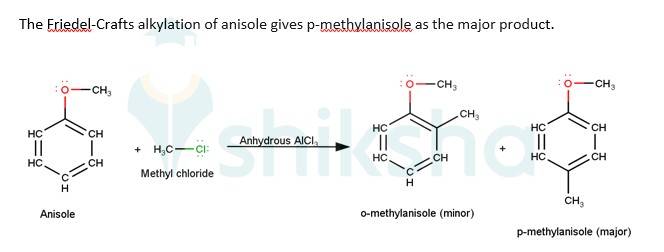
In case of anisole, methylphenyl oxonium ion, 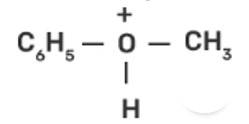
New answer posted
10 months agoContributor-Level 10
11.86

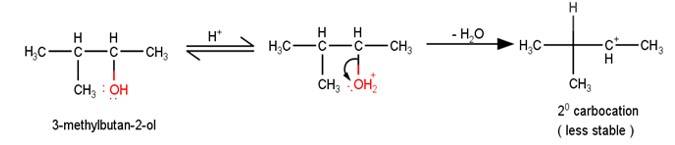
The first step in the mechanism of th e given reaction is protonation of the alcohol followed by loss of water to give a 20 carbocation.
e given reaction is protonation of the alcohol followed by loss of water to give a 20 carbocation.
2. The next step is a rearrangement of the 20 carbocations formed in the above step is less stable it rearranges by a 1,2-hydride shift to form more stable 3° carbocations.
New answer posted
10 months agoContributor-Level 10
11.85
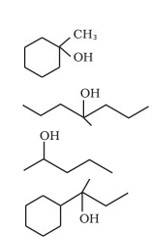
We know that the addition and elimination reactions are opposite of each other.Hence, for solving the above questions our approach should be to first dehydrate a suitable alcohol to give either a single alkene or a mixture of an alkene, if we obtain a mixture of alkene then we would have to detect which of the alkene will give us the desired alcohol. Wherever required the acid- catalyzed addition of water to alkenes will follow Markovnikov's rule.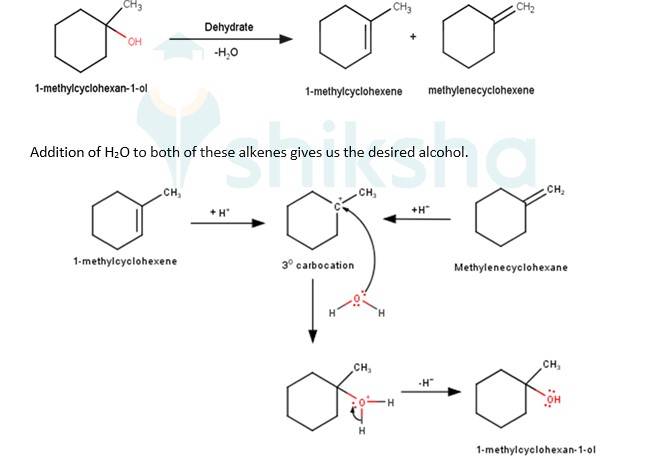
New answer posted
10 months agoContributor-Level 10
11.84
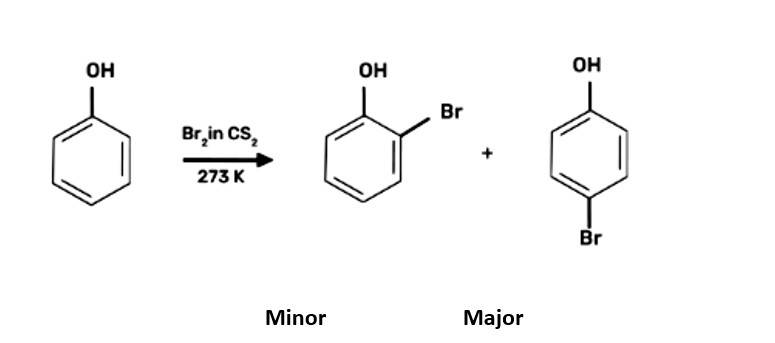
The driving force of all the reactions given to the question is that the alkoxy group is an ortho and para directing group because it exerts its +R effect in the benzene ring. Para position being comparatively more stable than the ortho position is usually preferred because ortho position leads to stearic hindrance, hence the major product is mostly the para- substituted compound.
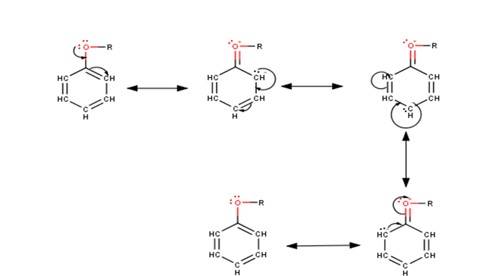
As seen from the resonating structures above the structure in which the negative charge is in the para position will form a more stable product when attacked by an electrophile. Hence in the following reactions, we will be considering that resonating stru
New answer posted
10 months agoContributor-Level 10
11.83
The reaction of HI with methoxymethane yields two different sets of products depending upon the initial amount of HI taken.
When equal moles of HI and methoxymethane are taken, a mixture of methyl alcohol and methyl iodide is
The mechanism is given below:
In the first step, methoxymethane reacts with hydrogen iodide to extract a proton to give the dimethyloxonium ion.
In the second step of the reaction, the Dimethyloxonium ion reacts with the iodide ion present to yield methyl iodide and methyl alcohol as the product via SN2 pathway.
If an excess of HI is used the methyl alcohol formed in Step II is also converted into methyl iod
New answer posted
10 months agoContributor-Level 10
11.82
(i) In aryl alkyl ethers the +R effect of the alkoxy group leads to an increase in the electron density of the benzene ring as they push electrons into the ring making the benzene ring activated towards electrophilic substitution reactions. This could be understood more clearly from the following resonating structures : -
(ii) It could be clearly seen from the above resonating structures that the electron density increases more at the ortho and para positions as compared to the meta positions. Hence, we can conclude that the alkoxy group directs the incoming substituents to ortho and para positions in the benzene ring.
For example
New answer posted
10 months agoContributor-Level 10
11.81
1-propoxypropane reacts with hydrogen iodide to give propan-1-ol and 1-iodopropane as the products.
2. Methoxybenzene reacts with hydrogen iodide to give phenol and iodomethane
Benzyl ethyl ether reacts with hydrogen iodide to give benzyl iodide and ethanol
New answer posted
10 months agoContributor-Level 10
11.80
The preparation of ether by acid dehydration of primary alcohol involves the nucleophilic addition of alcohol molecule to the protonated alcohol molecule as shown below: -
However, under these conditions secondary and tertiary alcohols forms alkenes rather than ethers. The reason for this being that due to stearic hindrance, nucleophilic attack by the alcohol molecule on the protonated alcohol molecule does not take place. Instead protonated 20 and 30 alcohols lose a molecule of water to form stable carbocations. The stable carbocations so formed prefers to lose a proton to form alkenes instead of forming ethers by undergoing
New answer posted
10 months agoContributor-Level 10
11.79
According to the question we have to perform the following conversion: -
The mechanism of the above reaction is as follows : - The mechanism is given below: -
In the first step, the alcohol gets protonated by the acid present to give a protonated alcohol.
In the second step, the nucleophilic attack of another alcohol molecule on the protonated alcohol gives us 1-propoxypropane as the desired product.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers