Alcohol Phenol And Ethers
Get insights from 261 questions on Alcohol Phenol And Ethers, answered by students, alumni, and experts. You may also ask and answer any question you like about Alcohol Phenol And Ethers
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The reagent used for the conversion of ethanol to ethanal is pyridinium chlorochromate (PCC) which is a complex formed by CrO3, pyridine and HCl which converts the primary alcohols to aldehydes.
CH3—CH2—OH ![]() CH3—CHO
CH3—CHO
Ethanol Ethanal
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
2-chloroethanol is more acidic than ethanol because the conjugate base of 2-chloroethanol is more stable than that of ethanol due to the -I effect of chlorine atom.
Stability: Cl—CH2—CH2—OΘ > CH3—CH2—OΘ
Acidity: Cl—CH2—CH2—OH > CH3—CH2—OH
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
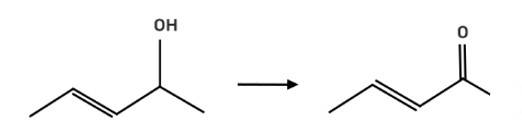
The reagent pyridinium chlorochromate (PCC) is a complex formed by CrO3, pyridine and HCl converts the secondary alcohol to ketone without oxidising the double bond.
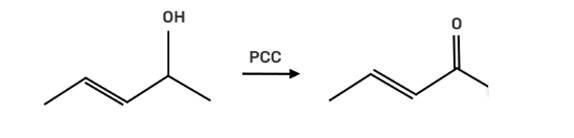
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Alcohols such as ethanol are made unfit for consumption by adding some additives like copper sulphate and pyridine which gives a foul smell, and bad taste to alcohol, it is known as denatured alcohol.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The solubility of alcohol in water depends upon the following factors:
(a) Hydrogen bonding: Alcohols form hydrogen bonding with water due to the presence of -OH group in alcohol and hence alcohols are soluble in water.
(b) Size of alkyl or aryl group: The alkyl and aryl group are hydrophobic in nature and larger the size of alkyl or aryl group of alcohol lesser will be the solubility of alcohol in water.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
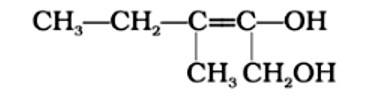
3-Methylpent-2-ene-1,2-diol
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
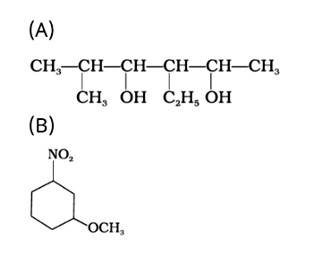
The IUPAC name of the given compound is:
(A) 3-Ethyl-5-methylhexane-2,4-diol
(B) 1-Methoxy-3-nitrocyclohexane
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The structure of glycerol is:
CH2—CH—CH2
| | |
OH OH OH
The IUPAC of glycerol is Propane-1,2,3-triol.
New answer posted
6 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Commercially, ethanol (C2H5OH) is made by fermenting carbohydrates, which is the earliest process. In the presence of an enzyme called invertase, sugar in molasses, sugarcane, or fruits like grapes is transformed to glucose and fructose (both of which have the formula C6H12O6).
In the presence of another enzyme, zymase, present in yeast, glucose and fructose are fermented.
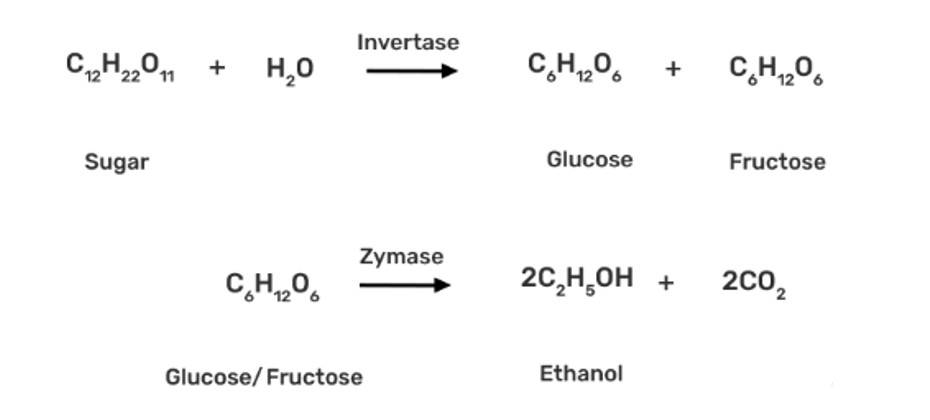
Grapes are used to make wine because they contain sugars and yeast. The amount of sugar in grapes increases as they ripen, and yeast forms on the outer peel. When grapes are crushed, sugar and enzymes c
New answer posted
6 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
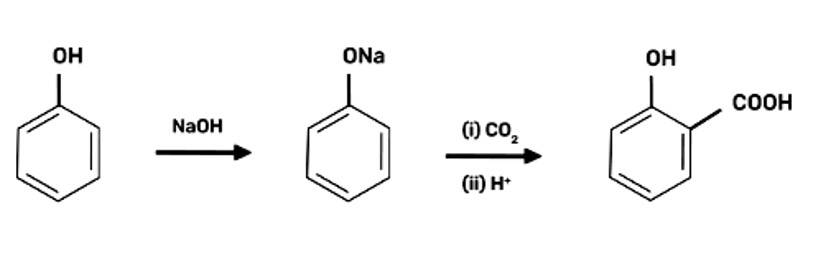

2-Hydroxybenzoic acid
&nb
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers