Aldehydes, Ketones and Carboxylic Acids
Get insights from 184 questions on Aldehydes, Ketones and Carboxylic Acids, answered by students, alumni, and experts. You may also ask and answer any question you like about Aldehydes, Ketones and Carboxylic Acids
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
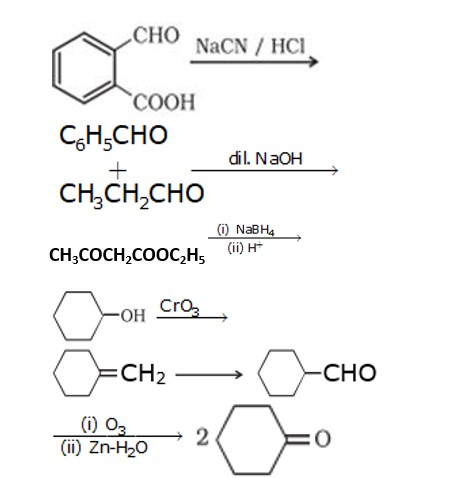
1. Cyclohexanone forms cyanohydrin in good yield because the ketonic group has very less steric hindrance at both the ortho position but 2,2,6 tri methyl cyclohexanone have high steric hindrance which reduces the attack from CN nucleophile.
New answer posted
7 months agoContributor-Level 10
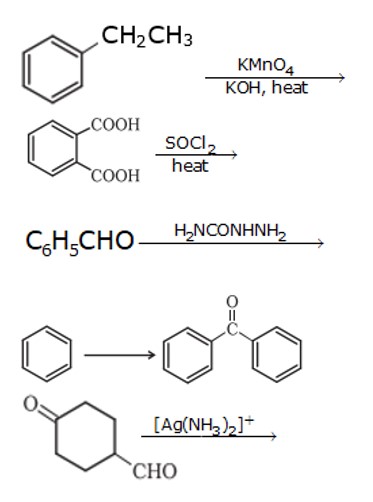
1. When 1- phenyl ethane is oxidized with a strong oxidizing agent like KMnO4, it forms a benzoic acid ion.
New question posted
7 months agoNew question posted
7 months agoNew answer posted
7 months agoContributor-Level 10
1. Acetylation refers to the process of introducing an acetyl group (resulting in an acetoxy group) into a compound, namely the substitution of an acetyl group for an active hydrogen atom. A reaction involving the replacement of the hydrogen atom of a hydroxyl group with an acetyl group (CH3CO) yields a specific ester, the acetate. Acetic anhydride is commonly used as an acetylating agent reacting with free hydroxyl groups., this reaction is usually carried out in the presence of base like pyridine.

2. Aldeydes having no α-H undergo the disproportion reaction in the presence of Strong alkali, it is a chemical reacti
New answer posted
7 months agoContributor-Level 10
1. When propanone reacts with NaBH4 it will form propan-2-ol.This alcohol is dehydrated to form propene.
2. When benzoic acid is treated with SOCl2 it chlorinates the acid. After controlled hydrogenation it forms benzaldehyde
3. When ethanol is treated with Cu at 573 k, it will oxidize to ethanal. When ethanal is treated with Dil.NaOH it will form 3-Hydroxy butanal
New answer posted
7 months agoContributor-Level 10
1. When benzene is treated with Br2 In presence of ferric bromide (brominating agent) they form bromobenzene. When bromobenzene is treated with Mg in ether it will form Grignard reagent, and if the CO2 is treated with Grignard reagent (in acidic condition) it will form benzoic acid. After esterification reaction in presence of methanol it will form methyl benzoate.

2. When benzene is treated with Br2 In presence of ferric bromide (brominating agent) they form bromobenzene. When bromobenzene is treated with Mg in ether it will form Grignard reagent, and if the CO2 is treated with Grignard reagent (in acidic condition) it will f
New answer posted
7 months agoContributor-Level 10
a )Tollen's test –Due to oxidizing nature of aldehydes they get easily oxidized, wheareas in case of ketones they are not readily
oxidizable. And tollens test exploits this fact, [Ag (NH3)2]+ is used as reagent Ag mirror is formed during this reaction
New answer posted
7 months agoContributor-Level 10
1. Di-tertbutyl, ketone 2. +I effect donates e- . Br group will show +I effect along the chain but as I effect is distance dependent effect it will die as the distance increase.+I effect of alkyl group will reduce the acidity of a compound whereas –I effect will increase the acidity, I effects are distance dependent, correct order will be (CH3)2CHCOOH< CH3CH2CH2COOH< CH3CH (Br)CH2COOH < CH3CH2CH (Br)COOH. 3. As we know the electron releasing groups (ERG) reduces the acidic strength of the compound (via inductive effect) whereas the electron withdrawing group (EWG) will increase the acidic strength of compound, and methoxy group is
New answer posted
7 months agoContributor-Level 10
ANSWER: Given compound is having 8 carbons, and on reaction with chromic acid C is converted back into B, as chromic acid reaction couldn't add any carbon hence both alcohol and acid must contain 4 carbons and it is given in the question that on dehydration C will give but-1-ene so alcohol will be butan-1-ol (C) and acid will be butan-1-oic acid (B) and given reaction will be ester hydrolysis,
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers