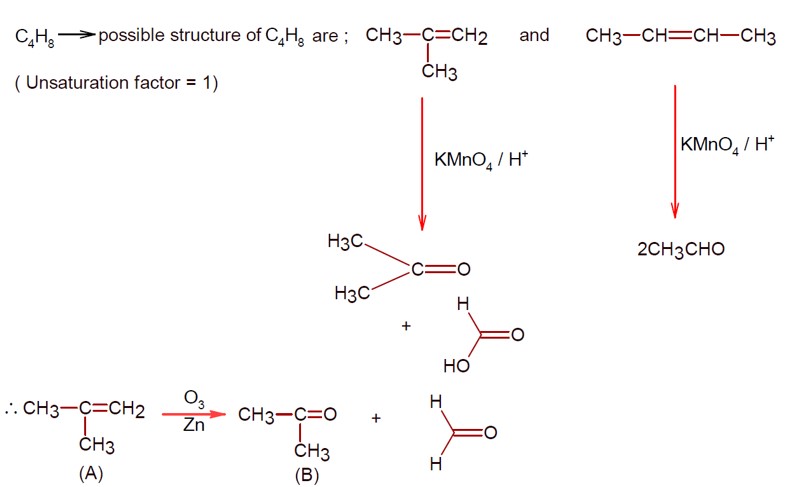
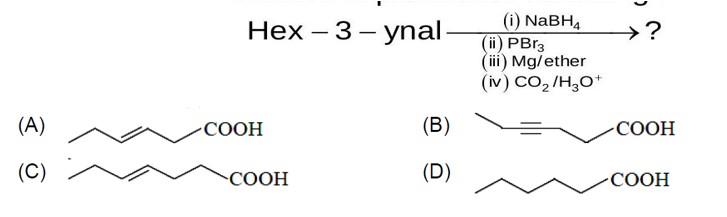Alkenes
Get insights from 10 questions on Alkenes, answered by students, alumni, and experts. You may also ask and answer any question you like about Alkenes
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
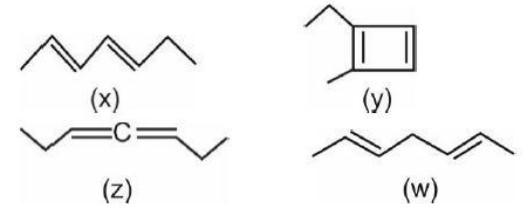
Stability order :
Conjugate > Isolated > Cumulative and (y) is Anti aromatic.
HOC ∝ 1 / (Stability of Alkene)
New answer posted
4 months agoContributor-Level 10
Kolbe's electrolysis is a process where an aqueous solution of sodium and potassium salt undergoes electrolysis to lead to the formation of a symmetrical alkene. Principle of this technique is that at anode, the carboxylate ion undergoes oxidation and loses carbon dioxide (CO? ). Similarly, at cathode the water decomposes to form hydrogen gas. The alkyl radicals formed in these two cases combine to form alkenes.
New answer posted
4 months agoContributor-Level 10
According to this rule, when a hydrogen halide (HX) or water is added to an asymmetrical alkene, the hydrogen atom (H) gets attached to the carbon atom of the double bond which has more hydrogen atoms, and the halide (X) or OH group attaches to the carbon atom which has fewer hydrogen atoms.
New answer posted
4 months agoContributor-Level 10
The most common method used for the identification of alkenes is the bromine water test. We simply have to add a few drops of bromine water to the compound which is to be identified. If the compound actually is an alkene, the reddish brown colour will get decolorized (? bond reacts with bromine and breaks the double bond).
New question posted
5 months agoNew question posted
5 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers